Modern Treatment Paradigms in EGFR-Mutant Advanced NSCLC: An Opinion Editorial
In recent years, the landscape of advanced non–small cell lung cancer (NSCLC) therapy for patients with EGFR mutations has undergone a significant transformation. As an editor who has closely followed developments in oncology, I have observed that our approach to treating this challenging condition has shifted from relatively straightforward monotherapies to increasingly complex combination regimens. In this editorial, I will offer my perspective on the current state of treatment strategies, the balance between efficacy and toxicity, and the importance of personalized care based on patient-specific factors.
Drawing on the insights shared by medical and thoracic oncologists during a recent workshop held in conjunction with the 2025 Annual Meeting of the American Society of Clinical Oncology, it is clear that the conversation around EGFR-mutant NSCLC is filled with both promise and challenges. The discussion was rich with practical advice on how to manage tricky parts such as balancing the improved outcomes of combination therapy with its often intimidating side effects, and how to figure a path through patient-specific considerations.
Combination Therapy Versus Monotherapy in First-Line Treatment
One major facet of the conversation in academic and cancer specialty circles revolves around the decision between using osimertinib as a monotherapy versus adopting a combination approach early on. Data from prominent trials like FLAURA2 and MARIPOSA have sparked intense debate over whether combining targeted agents, such as amivantamab and lazertinib, delivers significantly better outcomes than monotherapy while still maintaining an acceptable safety profile.
A recurring viewpoint among the expert panel is that, for many patients, osimertinib remains a robust option given its known efficacy as a single agent. However, as we witness evolving trends, combination regimens are increasingly becoming the default choice, especially in cases where patients present with additional tricky parts such as frailty, brain metastases, or the presence of uncommon co-mutations. In these situations, the slight differences in response between monotherapy and combination therapy underscore a need for personalized strategies that put patient quality of life front and center.
Understanding Trial Data: FLAURA2 and MARIPOSA
A closer look at the FLAURA2 and MARIPOSA trials illustrates the fine points of our current treatment approach. The FLAURA2 regimen, which typically involves an osimertinib-based scheme that sometimes incorporates chemotherapy, is favored by many due to its balance between overall survival benefits and a relatively manageable toxicity profile. In contrast, the MARIPOSA regimen, which relies on a combination of amivantamab and lazertinib, offers a promising efficacy profile, yet its administration comes with a set of tangled issues.
Clinicians have noted that the MARIPOSA strategy can be nerve-racking due to the extra care needed in managing its associated side effects, particularly the toxicities of amivantamab. The discussion at the workshop pointed out that managing these issues often involves dose and frequency adjustments—a practice that some experts believe might be substantially improved in the future with the introduction of subcutaneous (SC) administration.
Comparison of Therapeutic Benefits and Toxicity
When dissecting the results from clinical trials, it becomes apparent that every regimen carries its own set of advantages and hidden complexities. While combination therapies may promise enhanced efficacy over time, they also introduce more complicated pieces when it comes to side effect management. This is especially true with the amivantamab-lazertinib combination, where increased overall survival benefits must be carefully weighed against the likelihood of treatment interruptions due to toxicities.
In my opinion, no approach is without its compromises. For instance, the FLAURA2 approach appears to offer better quality of life for many patients by avoiding some of the nerve-racking and off-putting side effects sometimes seen in combination regimens. For oncologists, the challenge is to strike a balance that preserves the quality of life while still pushing for therapeutic gains.
| Regimen | Efficacy | Safety Profile | Clinical Considerations |
|---|---|---|---|
| Osimertinib Monotherapy/Osimertinib + Chemotherapy (FLAURA2) | Proven overall survival benefit | Manageable toxicity, suitable for many patients | Preferred when balancing treatment benefits with side effects |
| Amivantamab + Lazertinib (MARIPOSA) | Emerging data supports improved efficacy | Higher risk of toxicities requiring careful monitoring | Consider combination therapy in high-risk patients |
Balancing the Efficacy-Toxicity Equation
It is important to get into the nitty-gritty of balancing therapeutic benefits against the additional burdens of toxicity. The discussion among oncologists highlighted that the additional side effects associated with combination regimens tend to be both overwhelming and sometimes intimidating. For example, amivantamab, a key component of some combination therapies, can sometimes have dosing and administration challenges that impact patients’ daily lives.
One common strategy that specialists have adopted involves starting patients at a dose lower than the maximum approved dosage and extending the interval between sessions from every 2 weeks to every 3 weeks. This approach helps to reduce the intensity of side effects and can make the treatment regimen less off-putting for patients who might otherwise be deterred by the prospect of frequent toxicity monitoring.
Strategies to Ease Toxicity Concerns
Reducing the intensity of side effects is not simply about lowering the dose; it’s also about managing the treatment schedule and ensuring that the regimen is tailored to individual patients’ needs. For many clinicians, the key is to manage the delicate balance between the expected improved survival and the manageable side effects of the treatment. Here are some strategies that have been discussed:
- Dose titration: Initiating treatment at a reduced dose allows the patient’s body to adjust, potentially mitigating overwhelming side effects before gradually increasing to the optimal therapeutic level.
- Extended dosing intervals: Shifting from a bi-weekly to a tri-weekly schedule has been found to improve tolerability without substantially sacrificing the therapeutic benefits.
- Implementation of prophylactic measures: Clinics may spend significant time (sometimes up to an hour) preparing patients regarding strategies to preemptively manage side effects, particularly dermatologic issues associated with the combination.
These strategies, while perhaps adding a layer of complexity to treatment planning, are crucial for ensuring that the benefits of novel therapies are not overshadowed by their side effects.
Personalized Medicine: Factoring in Patient-Specific Characteristics
A recurring theme in the expert discussions is that the treatment approach must be highly personalized. When deciding between osimertinib monotherapy and combination treatment strategies, patient-specific factors play a central role. Attributes such as frailty, the presence of brain metastases, kidney function, and even socioeconomic factors significantly impact which regimen is most suitable.
For patients who present with two or more risk factors—such as unusual co-mutations or impaired hematologic conditions—combination therapy might very well be the preferred option. Conversely, if a patient exhibits fewer complicated pieces of clinical risk or smaller distinctions in their overall condition, a more straightforward monotherapy approach may be justified.
Key Considerations in Personalized Treatment
A few key points arise when tailoring treatment for the individual:
- Frailty and Co-Mutations: Patients who are considered frail or who have additional genetic alterations may not tolerate full-dose combination therapy well. In these cases, the benefit-to-risk ratio of adding another agent must be carefully considered.
- Organ Function: Impaired kidney function and certain hematologic conditions can affect how a patient metabolizes and responds to therapy, making dose adjustments and therapeutic sequencing crucial.
- Socioeconomic Factors: The availability of resources like infusion slots, supportive medications, and specialized nursing support can affect which treatment paradigms are feasible. In environments where clinical trial support is limited, a simpler regimen may be more practical.
In many ways, this is a call to move beyond a one-size-fits-all approach and design treatment plans that account for both the science and the individual challenges of each patient.
Sequencing Strategies Post-Progression: The Second-Line Conundrum
There is a common sentiment among experts that, despite significant progress with first-line therapy, managing patients who experience disease progression remains full of problems. Once a patient progresses after initial treatment—often with frontline osimertinib—oncologists are confronted with an array of options, each with its own set of tricky parts.
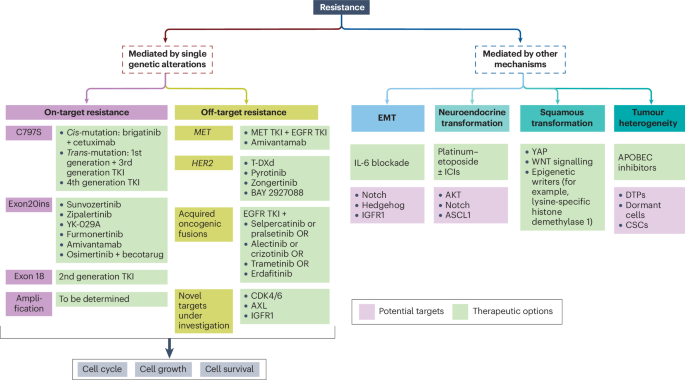
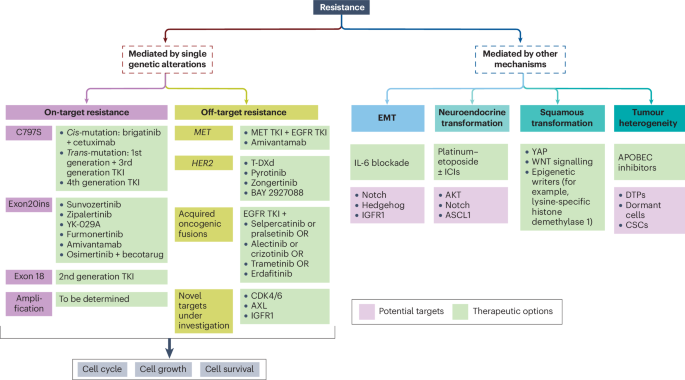
Second-line treatment strategies are evolving to become more refined and biomarker-driven. When faced with disease progression, oncologists are increasingly relying on tissue-based assessments, such as MET IHC tissue testing, to detect new fusions or MET amplification. This helps them to decide whether to shift the treatment strategy to MET tyrosine kinase inhibitors or to stick with a modified version of the existing regimen.
Biomarker-Guided Second-Line Therapies
In the era of personalized treatment, identifying fine shades of resistance is critical. Clinicians have noted that one of the most challenging and tangled issues is the emergence of resistance mutations to first-line therapies. For example, even when patients exhibit stable or minimal brain metastases, some oncologists consider continuing osimertinib post-progression. However, if the results of trials like COMPEL are negative, there is a growing tendency to reconsider continuing osimertinib in such circumstances.
Moreover, the integration of immunotherapies into treatment regimens adds another layer of subtle details. Some experts indicate that the possibility of employing PD-1 inhibitors like ivonescimab in combination or prior to restarting EGFR-blockade agents (such as osimertinib) raises both optimism and concerns. The issue here is that removing the EGFR blockade in patients with brain progression might trigger rapid withdrawal flares, which is a scenario oncologists are keen to avoid.
Treatment Accessibility and Practical Implementation Challenges
Even the most promising treatment regimens can be stalled by practical obstacles. Issues such as obtaining infusion slots, securing the necessary pharmaceutical supplies, and effectively managing supportive medications are mentioned as potential barriers in fully implementing the MARIPOSA regimen. These practical hurdles, though seemingly mundane in comparison to the high-stakes decisions about patient care, have a profound impact on treatment timelines.
What is particularly challenging is that these obstacles are not uniform across all healthcare settings. In well-resourced academic centers, there may be dedicated clinical trial support and specialized nursing staff who can patiently explain a complex prophylaxis regimen to patients. In contrast, community oncologists without such resources might struggle with these added time constraints, thereby slowing the adoption of combination therapies.
Addressing the Bottlenecks in Clinical Practice
There are several ways the oncology community can work through these tangled issues:
- Development of Starter Packs: The idea of creating starter packs that include lazertinib and subcutaneous amivantamab is particularly appealing. These packs could streamline the process of initiating combination therapy, reducing delays and minimizing scheduling issues.
- Enhanced Patient Counseling: While spending an hour counseling each patient might be feasible in specialized centers, finding a more efficient way to deliver this information could be critical in community settings. Digital platforms and educational videos might help fill this gap.
- Clearer Guidelines: More transparent and accessible guidelines on managing dose adjustments and side effects would help clinicians feel more comfortable with the newer regimens. This would ensure that the availability of effective treatments is maximized and that patients receive timely care.
In my view, bridging the gap between clinical trial results and real-world practice is one of the most pressing challenges. Without proactive measures to address these practical bottlenecks, the best treatment strategies may not be fully realized in everyday clinical settings.
Managing Toxicities: Dose Adjustments and Future Possibilities
Another discussion point that continues to engage the oncology community is how best to manage the side effects related to combination therapies. Amivantamab, although a promising agent, is notorious for its side effect profile. Many oncologists have resorted to tactics such as initiating treatment at a reduced dose and then gradually increasing the dose as the patient adapts. Adjusting the frequency of administration—from every 2 weeks to every 3 weeks—has also shown promise in alleviating some of the more overwhelming side effects.
Looking ahead, many experts are excited by the potential of subcutaneous (SC) administration of treatments like amivantamab. SC formulations could ease many of the current challenges, making it easier to figure a path through the treatment regimen without the nerve-racking need for constant adjustments. This could be a game changer, especially for communities that lack sufficient clinical trial infrastructure. It also stands to improve patient quality of life, which remains a key consideration in treatment decisions.
Future Directions in Treatment Administration
As the field of oncology continues to evolve, so too does the approach to drug administration. Here are a few potential future directions that hold promise:
- Subcutaneous Administration: Moving to an SC form of amivantamab could reduce the logistical complications associated with intravenous infusions. It also has the potential to provide a more predictable absorption profile, translating into more consistent therapeutic outcomes.
- Tailored Dose-Titration Protocols: Ongoing research aims to refine dose titration protocols to better match individual patient tolerability. These protocols could incorporate predictive biomarkers, which in turn would allow clinicians to better forecast which patients are likely to experience more severe side effects.
- Digital Monitoring Tools: With the rise of telemedicine and digitized patient monitoring, future regimens might leverage digital tools to monitor side effects in real time. This could allow clinicians to make quicker, more informed decisions about dose adjustments.
Such innovations would not only improve the therapeutic index of combination therapies but also help reduce the overarching anxiety among patients and clinicians alike.
Biomarkers: The Compass to Predicting Resistance and Guiding Therapy
As progress in treatment continues, biomarkers are emerging as an essential tool for predicting resistance and guiding the sequence of therapies in EGFR-mutant NSCLC. When oncologists are tasked with working through the tricky parts of deciding the next steps after disease progression, tissue-based assays like MET immunohistochemistry (IHC) have proven to be key. These tests can reveal the hidden complexities of resistance mechanisms, such as MET amplification or the emergence of new fusion partners.
The push towards biomarker-guided therapy is driven by a desire to make second-line interventions more targeted and effective. When a new fusion or MET amplification is discovered, the post-progression therapeutic strategy may shift towards incorporating MET tyrosine kinase inhibitors—a move that should ideally be guided by predictive markers. This evolution in practice underscores the importance of additional tools that help clinicians find their way through treatment resistance.
How Biomarkers Improve Patient Care
Biomarkers offer several advantages in managing EGFR-mutant NSCLC:
- Predictive Value: Biomarkers can help forecast which patients might be at a higher risk for developing resistance, letting clinicians adopt preemptive strategies rather than reacting to disease progression.
- Therapy Customization: With clear guidance from biomarker assays, treatments can be tailored to target the specific mechanisms driving resistance, ultimately enhancing treatment efficacy.
- Optimized Sequencing: By identifying the fine shades that differentiate one patient’s resistance profile from another, oncologists can more accurately time the switch between therapies or opt for combination courses that hold more promise in later lines of therapy.
In my opinion, the integration of biomarkers into routine clinical practice is a must-have in oncology. Such an approach not only refines the art of treatment sequencing but also provides a critical perspective when assessing the risk–benefit equation tied to therapy decisions.
Recommendations for Maximizing Therapy Accessibility
While the evolving treatment landscape for EGFR-mutant advanced NSCLC is filled with promise, there remain several practical recommendations to improve accessibility and streamline the clinical process. One of the most pressing issues today is the need for starter packs that contain all the necessary components to initiate combination regimens quickly. Such starter packs would reduce delays that currently stem from issues like scheduling infusion slots and procuring supportive medications.
I believe that a coordinated effort among pharmaceutical companies, healthcare providers, and regulatory bodies is key to addressing these on edge challenges. By ensuring that compassionate access programs and starter kits are widely available, the healthcare system can find its way toward enhanced delivery of care and better overall patient outcomes.
Key Recommendations for Clinical Practice
- Introductions of Starter Packs: Packaging lazertinib and SC amivantamab together would streamline treatment initiation and reduce waiting times.
- Enhanced Biomarker Testing Accessibility: Ensuring that MET IHC and other biomarker assays are readily available in community settings would help tailor second-line treatment regimens more effectively.
- Clearer Supportive Care Guidelines: The development and dissemination of standardized protocols for managing the side effects of combination therapies can make these regimens more approachable, especially outside major academic centers.
- Digital Health Innovations: Introducing telehealth platforms for real-time side effect monitoring and dose adjustment advice can help bridge the gap between trial settings and everyday practice.
These recommendations are not mere suggestions; they are critical steps that must be taken to ensure that innovative therapies do not remain confined to clinical trials but are fully integrated into routine patient care.
Final Thoughts: The Future of EGFR-Mutant NSCLC Therapy
The discussions and debates emerging from recent expert meetings reflect a field that is both active and rapidly evolving. While combination therapies, such as the MARIPOSA regimen, offer promising new avenues for improving overall survival, they also introduce a series of tricky parts that require careful navigation. On the other hand, more established regimens like FLAURA2 provide a well-trodden path that balances efficacy with a manageable safety profile.
From my point of view, the future of EGFR-mutant advanced NSCLC therapy lies in striking the optimal balance between maximizing therapeutic benefit and minimizing the intimidating side effects that can make treatment so nerve-racking. This will only be achieved through a combination of continued clinical research, effective integration of biomarkers, and pragmatic efforts to make therapies accessible in a variety of healthcare settings.
As we look ahead, one thing remains clear: every patient is unique, and our treatment paradigms must be equally flexible. In practice, this means that oncologists must work through the tangled issues using all the tools at their disposal—including lower starting doses, extended dosing intervals, and biomarker guidance—to ensure that patients receive the most effective treatment possible with the least disruptive side effects.
Moreover, the industry’s push towards innovations like subcutaneous formulations and digital health monitoring represents a promising step forward in reducing the overwhelming side effects and confusing bits of current treatment regimens. If these advances can be successfully implemented, the future may hold a less burdensome experience for patients and a more streamlined process for healthcare providers.
In Summary
To summarize, the evolution of treatment for EGFR-mutant NSCLC is marked by several key points:
- Emergence of Combination Therapy: Although promising in improving outcomes, combination regimens such as MARIPOSA come with additional, sometimes overwhelming side effects that must be intelligently managed.
- Balancing Act: The core challenge lies in balancing the enhanced efficacy of combination treatment with the necessity to maintain a patient’s quality of life. This often involves careful dose adjustments and scheduling tweaks.
- Personalized Therapy: Every patient presents with a mix of factors—frailty, co-mutations, organ function issues, and socioeconomic constraints—that require a tailored approach. One-size-fits-all does not apply in this intricate field.
- Biomarker Integration: Using biomarkers to predict resistance and guide subsequent treatment choices is no longer optional but a critical, key component of modern oncology.
- Practical Implementation: Addressing obstacles such as infusion scheduling, medication procurement, and patient counseling will determine how widely and effectively these treatments are adopted.
It is an exciting time in the field of oncology. As research continues to provide us with finer details and better pathways for treatment, the ultimate beneficiary will be the patient. By proactively addressing both the theoretical and practical challenges, we can ensure that the strides made in the laboratory quickly translate into improved survival rates and better quality of life in the clinic.
In closing, I urge my colleagues, policymakers, and industry leaders to invest not only in innovative compounds and groundbreaking clinical trials but also in the practical infrastructure and educational initiatives that will support these advances. Only through a comprehensive approach can we truly transform how we treat EGFR-mutant advanced NSCLC and help our patients navigate the often intimidating twists and turns of cancer therapy.
The future promises more refined treatment protocols that are both super important and patient-centered. With continued collaboration, the field of oncology will undoubtedly move toward smoother, more effective treatment pathways that take into account every delicate detail—from the subtle parts of drug side effects to the fine shades of individual patient resistance.
This is a pivotal moment in the journey toward more personalized cancer care. As we move forward, let us work together—clinicians, researchers, and industry partners alike—in designing therapies that not only extend life but also preserve the quality of life, ensuring that each patient feels supported every step of the way.
With these strategies in place and a commitment to ongoing innovation, the treatment of EGFR-mutant advanced NSCLC will no longer be viewed as a series of tangled issues but as a testament to the power of personalized medicine and the resilience of the human spirit in the face of a challenging disease.
Originally Post From https://www.onclive.com/view/addressing-the-most-critical-treatment-questions-in-egfr-mutant-advanced-nsclc
Read more about this topic at
Advancing EGFR Therapy in Non-Small Cell Lung Cancer
Evolving treatment for advanced Non-small cell lung …