Early Warning Signs for Ovarian Cancer: An Opinion Editorial
Recent breakthroughs in ovarian cancer research provide fresh perspectives on a disease with many tangled issues. Experts at Mayo Clinic have uncovered what they believe to be some of the earliest cellular indicators of this life‐threatening condition. In an opinion editorial format, we take a closer look at these findings, the innovative research tools used to generate them, and how these discoveries might reshape preventative strategies for ovarian cancer in the years ahead.
The research involved a collaboration among physicians, scientists, and patients working together to figure a path through the challenging realm of early cancer detection. With ovarian cancer typically detected only after it has spread beyond the ovaries, this new work has ignited hope that early signs of the disease can be spotted before it reaches an overwhelming stage.
Understanding the Ridged Challenges of Ovarian Cancer Diagnosis
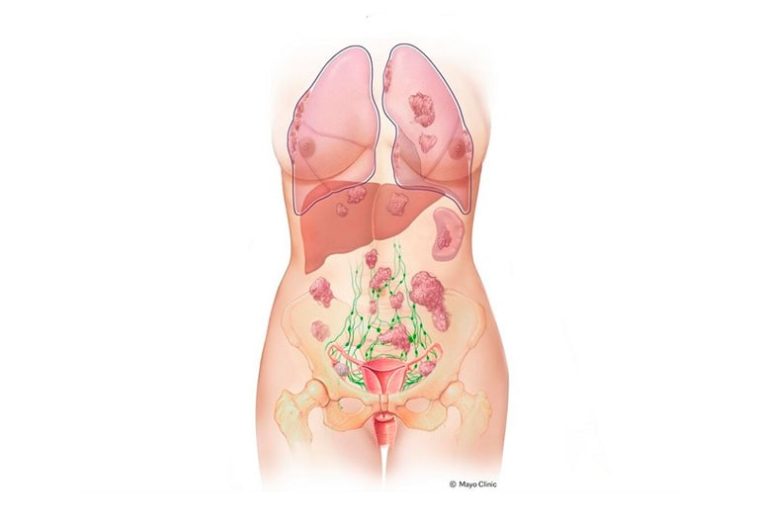
Ovarian cancer is notorious for its nerve-racking presentation. Approximately 75% of cases are only diagnosed once the cancer has advanced to stage 3 or 4. That means by the time the disease is discovered, it has already moved beyond the ovaries, making treatment more challenging and prognosis less favorable. This daunting reality has led researchers to dig into the early, hidden changes that may signal the onset of such cancers.
Historically, the tricky parts of ovarian cancer detection stem from the absence of obvious symptoms and the limited availability of early diagnostic tests. It is these confusing bits of the disease’s progression that make it so loaded with problems. With research now shining a light on the very beginning of cancer development—specifically in the fallopian tubes—there is renewed optimism for early intervention strategies.
Uncovering Early Cellular Signs: The Case That Sparked a Breakthrough
A striking patient case at Mayo Clinic set the stage for these important findings. A 22-year-old woman, known to be at high risk due to hereditary BRCA2 and TP53 mutations, visited the clinic with a complex medical history. These particular gene mutations are associated with conditions such as hereditary breast and ovarian cancer syndrome and Li-Fraumeni syndrome—both known to significantly increase the risk of several cancers.
During her health evaluation, the patient was found to have breast cancer and an ovarian cyst, which later turned out to be benign. Given her extraordinary risk, she made the brave decision to undergo a bilateral salpingo-oophorectomy—a surgical removal of her ovaries and fallopian tubes, along with a mastectomy and hysterectomy. It was during the analysis of her removed tissue that researchers identified subtle cellular abnormalities, suggesting early warning signs of ovarian cancer far ahead of any visible symptoms.
Innovative Laboratory Techniques: A Closer Look at Single-Cell Technologies
One of the game-changing approaches in this research was the use of single-cell RNA sequencing. This state-of-the-art genomic analysis tool provided deep insights into the minute details of epithelial cell development. Researchers were able to figure a path through the hidden complexities of the fallopian tube cells, noting that secretory cells appeared disproportionately to multiciliated cells. Multiciliated cells typically help move the fertilized egg along the tube, while secretory cells produce fluids crucial for embryonic nourishment.
In this high-risk patient’s tissue, the secretory cells not only outnumbered the multiciliated ones, but they also seemed to drive chronic inflammation—one of the known factors that can trigger cancer development. Such findings prompt us to get into the subtle details of cancer’s early progression and may eventually pave the way for early diagnostic tools that operate before the disease becomes full of problems.
Living Tissue Biobanks and Organoid Models: Bridging Laboratory Research and Clinical Application
In an effort to replicate and further explore these early cellular changes, Mayo Clinic researchers have established a living fallopian tube biobank. This resource is built from cells and tissues donated by patients and is designed to help scientists dive in to study the origins of ovarian cancer using real human samples. These samples are then used to grow organoids, which are essentially tiny, three-dimensional models of the cells and tissues found in the fallopian tubes.
Such organoid systems offer a miniaturized and controllable way to replicate human cellular behavior. They allow researchers to poke around the fine points of cell development under conditions that closely mimic those in the human body. This innovative tool is critical for understanding and addressing the subtle parts of ovarian cancer’s development in a step-by-step manner.
Key Advantages of a Living Fallopian Tube Biobank
- Authenticity: Directly derived from patient samples, enhancing the real-world relevance of the studied models.
- Diverse Representation: Incorporating tissues from individuals with varying levels of ovarian cancer risk, which helps account for the small differences in genetic background.
- Precision in Testing: Allowing researchers to perform single-cell analyses and observe changes at an incredibly detailed level.
- Innovation in Drug Screening: Providing platforms to test new prevention and treatment strategies before moving to clinical trials.
The creation of such a resource demonstrates how modern biotechnology can sort out the tricky parts of disease progression and offer key insights for preventive oncology.
Decoding Cellular Imbalances: The Role of Fallopian Tube Epithelial Cells
In a healthy fallopian tube, two primary types of epithelial cells coexist in a balanced environment. However, in the patient with high genetic risk, that balance was notably disrupted. The secretory cells overwhelmed the multiciliated cells, a shift that may signify an early warning sign of cancer risk. Moreover, these secretory cells were found to foster a state of chronic, low-grade inflammation known to be associated with cancer development.
This observation prompts us to take a closer look at how minor changes in the cell population can lead to larger, more daunting alterations later in the disease process. Researchers believe that similar cellular imbalances could serve as early targets for future screening methods and prevention strategies.
Comparing the Cellular Environment: A Summary Table
| Cell Type | Normal Function | Observations in High-Risk Tissue |
|---|---|---|
| Multiciliated Cells | Transport fertilized eggs through the tube | Significantly reduced numbers |
| Secretory Cells | Provide nourishment and protection | Excessively increased numbers, driving chronic inflammation |
This table simplifies some of the hidden complexities, helping us visualize the small distinctions that could have a large impact on understanding ovarian cancer’s origins.
Single-Cell Insights: Unraveling the Early Triggers
The use of advanced single-cell technologies allowed the research team to trace cellular development in unprecedented detail. By breaking down the behavior of individual cells, scientists could see how the epithelial cells in the fallopian tubes were altered in a manner that might lead to ovarian cancer. This deep dive into the cellular microenvironment offers a clearer glimpse into the chain of events that lead to malignancy long before any signs come to light.
Dr. Nagarajan Kannan, a leading researcher involved in the study, emphasized that these findings could inform future strategies for early detection and precision prevention. Instead of waiting for ovarian cancer to become an overwhelming challenge, such approaches could eventually allow healthcare professionals to identify and intervene during the precancerous stage.
Personalized Prevention Strategies: Tailoring Screening and Interventions
One of the most promising aspects of this research lies in its potential to shape more personalized strategies for cancer prevention. Ovarian cancers, particularly the aggressive forms that originate in the fallopian tube, have been historically difficult to treat due to their tendency to present late. By understanding the fine shades and subtle parts of the disease’s early development, clinicians may one day be able to identify high-risk individuals with even greater accuracy.
Personalized prevention could include several key elements:
- Early and Frequent Screening: For individuals known to carry genetic mutations like BRCA2 or TP53, regular monitoring using advanced cell-based diagnostics might become a standard protocol.
- Customized Surgical Decisions: Decisions about preventative surgeries, such as prophylactic oophorectomy, could be improved by understanding each patient’s unique tissue profile.
- Tailored Medical Therapies: Patients might benefit from precision treatments that are developed specifically to target early-stage disease markers.
- Enhanced Patient Counseling: With better insight into the subtle details of disease progression, doctors could offer more personalized advice on both risk reduction and fertility planning.
These elements together form a multifaceted approach to tackling a disease that has long been a challenge to both doctors and patients alike. The goal is to find paths that reduce the chance of the disease progressing to an unstoppable stage.
Re-evaluating the Role of Hormones: Oral Contraceptives Under the Microscope
Intriguingly, the study found that the fallopian tube cells from the high-risk patient did not express progesterone receptor proteins. Oral contraceptives that contain progestins are widely understood to lower the risk of ovarian cancer by up to 50% in many women. However, the absence of these receptors in the patient’s tissue suggests that hormone-based prevention methods might not be universally effective. This finding challenges previous assumptions and reminds us that even well-established prevention strategies may face hidden twists when dealing with genetically complex cases.
Such observations push researchers to reconsider how different factors—genetic predisposition, cell receptor status, and hormonal influences—combine to affect ovarian cancer risk. It is a reminder that while oral contraceptives work as a super important tool in many situations, their effectiveness might be limited among certain high-risk populations. Ultimately, this knowledge could lead to more nuanced treatment and prevention plans that take into account small differences at the cellular level.
Future Directions: Investigating the Origins of Ovarian Cancer
The research team’s future plans include using the living fallopian tube biobank to poke around and better characterize exactly where ovarian cancer starts. By focusing on the very first changes in cellular behavior, scientists hope to map out the chain of events that lead from normal cell function to early precancerous signals and eventually to full-blown ovarian cancer.
The challenges ahead involve uncovering more of the tangled issues that contribute to the development of this aggressive disease. Future studies will likely compare the fallopian tube cellular landscape across a larger group of patients, including both high-risk individuals and those without known mutations. By doing so, researchers can identify consistent patterns that might eventually be targeted by new screening tools or pharmacological therapies.
Key Areas for Future Research
- Expanding the Biobank: Broadening the collection of patient-derived tissues to include samples from various demographic groups and different genetic backgrounds.
- Enhanced Genomic Profiling: Utilizing next-generation sequencing to track subtle changes in gene expression over time.
- Mapping Early Inflammatory Responses: Investigating the relationship between chronic inflammation in secretory cells and the initiation of cancer.
- Evaluating Hormonal Receptor Variability: Determining why some high-risk tissues lack progesterone receptors and how that affects prevention strategies.
By systematically addressing these questions, researchers aim to untangle the confused roadmap of ovarian cancer development, paving the way for more effective early detection and tailored interventions.
Bridging the Gap Between Laboratory Discoveries and Clinical Care
The findings from Mayo Clinic underscore the promise of translating laboratory research into clinically actionable insights. While the identification of early cellular changes in the fallopian tube represents a major step forward, much work remains before these insights can be integrated into routine practice.
At the moment, the potential of these discoveries is twofold. On the research side, they open up opportunities for developing new diagnostic tests that could detect ovarian cancer at its very inception. On the clinical side, they provide hope that future screening protocols might allow for interventions that prevent the full-blown onset of cancer. This dual approach of early detection and personalized prevention is exactly what the modern world of healthcare needs to tackle diseases with such nerve-racking outcomes.
Indeed, forging a strong bridge between innovative research and clinical application can help ensure that high-risk patients receive the super important care they need at the right time, potentially saving lives by addressing the disease before it has a chance to spread extensively.
The Patient Perspective: Emphasizing Hope and Empowerment
It is important to remember that behind every research paper and scientific breakthrough there is a patient story. The young woman whose case inspired these findings has, in many ways, become a beacon of hope for countless others who carry similar genetic risks. Her decision to take proactive steps, supported by cutting-edge medical care, represents the kind of patient empowerment that lies at the heart of modern healthcare.
From an editorial standpoint, this case encourages a rethinking of how we manage cancers that are full of confusing bits and daunting unknowns. It highlights the necessity of early identification and the promise of personalized medicine in reducing the overall burden of ovarian cancer. Empowered patients, along with dedicated clinicians and researchers, are key to ensuring that discoveries in the lab are translated into tangible benefits at the bedside.
Implications for Personalized Cancer Screening and Prevention
Personalizing healthcare is more than a buzzword—it is becoming a decisive factor in how diseases are detected and treated. With the revelations from this study, there is every reason to believe that ovarian cancer may eventually be caught at its inception in a manner tailored to an individual’s genetic makeup.
There are several aspects of personalized prevention that deserve attention:
- Genetic Testing: Widespread genetic screening for mutations such as BRCA2 and TP53 could help identify high-risk individuals long before symptoms develop.
- Customized Monitoring Protocols: In high-risk patients, regular and advanced cell-based diagnostics could be instituted to keep a close watch on early cellular shifts.
- Early Hormonal Assessments: Given the surprising findings regarding progesterone receptor absence in some tissues, further hormonal profiling may refine the use of preventive hormonal therapies.
- Patient Counseling and Informed Choices: More detailed assessments of individual risk could lead to more informed decisions regarding prophylactic surgeries and fertility planning.
This multi-pronged approach is designed to drain away the puzzling uncertainties that currently load ovarian cancer with so many nerve-racking outcomes, giving both patients and clinicians a clearer and more hopeful path forward.
Addressing the Hidden Complexity of Ovarian Cancer Origins
Ovarian cancer has often been regarded as a condition shrouded in mystery. The hidden cellular imbalances and fine details uncovered in recent studies invite us to reconsider previously held assumptions about where and how the cancer originates. The current evidence points toward the fallopian tubes as a key starting point for at least some forms of the disease.
As researchers continue to piece together the puzzle, it becomes increasingly obvious that ovarian cancer does not read the textbook. Instead, it develops through a series of subtle shifts in cell population and inflammatory processes that can occur long before traditional symptoms or diagnostic markers are evident.
This evolving understanding underscores the importance of advanced laboratory techniques—such as single-cell sequencing and organoid modeling—that can reveal the early, confusing bits of cancer genesis. By better comprehending these early triggers, the medical community may soon have the tools necessary to catch the disease at a stage when interventions are both more feasible and less invasive.
The Role of Interdisciplinary Collaboration in Advancing Cancer Research
Another key feature of this breakthrough is the close collaboration among specialists in genetics, oncology, pathology, and reproductive medicine. When faced with the brain-teasing challenges of evolving cell populations and hidden inflammatory processes, a team approach is essential to figure a path through the connected maze of implications.
Such collaboration is not only essential for interpreting laboratory data but also for implementing that information into effective clinical strategies. The collective expertise of a multi-disciplinary team allows the nuances of ovarian cancer development to be appreciated from multiple angles—a critical factor in making well-rounded clinical decisions that benefit patients.
This interdisciplinary approach also reinforces the importance of patient participation in research. With tissue donation and the sharing of personal medical histories, patients contribute directly to scientific progress. Their involvement transforms individual cases into a rich database of knowledge that continuously fuels new research directions and preventive strategies.
Balancing Optimism with Real-World Challenges
While these findings are indeed promising, they also remind us of the long road that lies ahead. The discovery of early cellular indicators is an essential first step, but moving from research to routine clinical use will require thorough validation, additional research, and careful integration into existing screening protocols.
There remain several complicated pieces that researchers must work through before early detection methods based on these findings can become widely available:
- Validation Across Diverse Populations: Early-stage tissue changes need to be confirmed in a broader group of patients before they can serve as reliable clinical markers.
- Development of Non-Invasive Testing Methods: While current research relies on tissue samples, future efforts must focus on creating less invasive diagnostic tools suitable for widespread screening.
- Cost and Accessibility: Advanced single-cell technologies and organoid models are currently expensive and require specialized expertise. Bridging this gap is crucial for broad implementation.
- Integration with Existing Protocols: Early detection methods will need to be harmonized with current clinical practices so that patients benefit from both innovative research and proven diagnostic techniques.
These challenges, though intimidating, should also instill hope. Each step forward in addressing these tangled issues brings us closer to a future where ovarian cancer can be managed before it reaches overwhelming, late-stage severity.
Conclusion: A New Era in Ovarian Cancer Prevention and Early Detection
In conclusion, the discovery of early cellular changes in the fallopian tubes of a high-risk patient marks a significant turning point in our understanding of ovarian cancer’s origins. With innovative technologies like single-cell RNA sequencing and living biobanks, researchers are now able to dive in to the very beginning of what may become a deadly disease. Such advances promise to lead to more personalized, effective strategies for early detection and prevention, potentially reducing mortality and improving the quality of life for countless patients.
There is no denying that the path forward is loaded with challenges. Yet, when we take a closer look at these early indicators and the potential for personalized intervention, we see a future where the management of ovarian cancer is less nerve-racking and more proactive. As interdisciplinary teams across the globe continue to work together, the hope is that the next few years will usher in a new era of tailored screening protocols, innovative therapies, and empowered patient decision-making.
This research not only underscores the importance of advanced genomic tools and laboratory techniques, but also highlights the essential role that patients play in shaping medical breakthroughs. With each donated tissue sample and every shared personal story, the medical community advances toward a more comprehensive understanding of ovarian cancer—a disease that has long been characterized by its overwhelming and often hidden onset.
Now more than ever, it is clear that our collective focus must be on transforming these early discoveries into everyday clinical practice. By integrating cutting-edge research with personalized patient care, we can withdraw some of the mystery from ovarian cancer. It is the responsibility of clinicians, researchers, and patients alike to work together in figuring a path through the tangled issues of early diagnosis and prevention. With every small step, we move closer to developing screening tools, risk-reduction strategies, and therapeutic interventions that will ultimately save lives.
As we continue to handle this formidable challenge, the message is one of cautious optimism. Every new study brings with it the potential for breakthroughs that will reshape our approach to cancer prevention. The key is to remain patient, persistent, and proactive—traits that have always defined the best in medical science. The lessons learned from early cellular changes in fallopian tube tissues remind us that the hidden complexities of cancer need not remain inscrutable forever. Instead, with modern research tools and dedicated interdisciplinary teamwork, we can uncover its secrets and fight back against one of the most nerve-racking cancers of our time.
In the end, the true victory will be measured not solely by the success in treating advanced disease, but by our ability to stop ovarian cancer in its tracks—long before it can spread and devastate lives. As we stand at the brink of this exciting new frontier, the integration of advanced genomic technologies, personalized patient care, and robust interdisciplinary collaboration promises a brighter, healthier future for those at risk. Let us embrace these technological and scientific advances, and work together to ensure that every twist and turn along the way brings us one step closer to defeating ovarian cancer.
Originally Post From https://scitechdaily.com/mayo-clinic-uncovers-first-warning-signs-of-ovarian-cancer/
Read more about this topic at
Early Warning Signs
Understanding The Early Warning Signs of Mental Illness