Revolutionary Cross-Therapy: When Cancer Medications Target Dementia
The boundaries between different fields of medicine continue to blur as scientists discover new ways to address old challenges. In a groundbreaking study led by a team at UC San Francisco and the Gladstone Institutes, researchers have found that two cancer medications may help reverse the twisting alterations that occur in the brain during Alzheimer’s disease. This unexpected approach not only offers fresh hope for a condition that affects millions but also highlights how exploring unconventional methods can lead to extraordinary breakthroughs in patient care.
The Unexpected Crossover: Cancer Drugs in Alzheimer’s Treatment
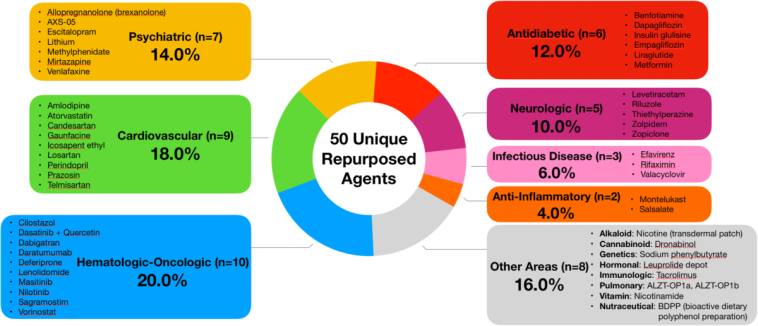
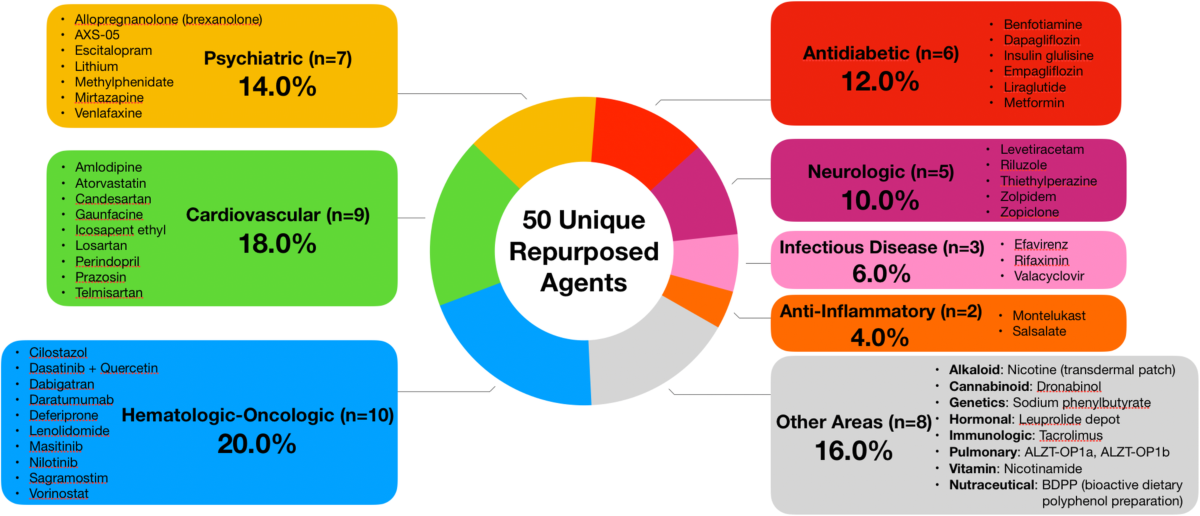
For decades, cancer medications have been used to fight malignant tumors by targeting rapidly dividing cells. Now, scientists are taking a closer look at these drugs to see if they can be repurposed to correct the tangled issues seen in Alzheimer’s. The study compared the gene expression signature of Alzheimer’s disease—a collection of changes in how genes are turned on or off within the brain—with the effects elicited by over 1,300 FDA-approved drugs. By aiming to reverse the specific gene expression changes, the researchers found a combination of two cancer medications that holds the potential to not only slow down but possibly reverse some of the cognitive impairments associated with Alzheimer’s.
Although it might seem off-putting to use cancer drugs to treat dementia, the rationale behind this approach is rooted in the recognition that many of the same gene pathways involved in uncontrolled cell growth can also impact brain cell function. The study offers a compelling case by presenting a detailed analysis of how these medications influence the neurons and supporting glial cells—cells that have been seriously impacted by Alzheimer’s disease.
A Closer Examination of the Research Approach
The researchers began by digging into the single-cell gene expression data from the brains of Alzheimer’s patients. This detailed analysis allowed them to piece together the fine points of how Alzheimer’s changes the way certain genes are expressed not only in neurons but also in glial cells, which help maintain the brain’s environment.
After establishing the altered gene expression profile, the next step was to figure a path through the database of approved drugs. By comparing the Alzheimer’s signature with drug-induced expression profiles, the team was able to identify candidates that produced opposite effects to those seen in the disease. This reverse-engineering approach is at the heart of computational drug repurposing, a method that leverages big datasets to find hidden connections between seemingly unrelated medical fields.
The study did not rely solely on laboratory data. The team also got into the electronic medical records of millions of patients to see how those who had taken some of these drugs for other health issues fared in terms of developing Alzheimer’s. This multifaceted exploration provided a more comprehensive view of the drugs’ potential benefits and added an extra layer of validation to the findings.
Step-by-Step: How Researchers Found the Link
Breaking down the study into understandable pieces shows how careful and methodical the approach was. Here are the main steps the researchers took:
- Analyzing Gene Expression: Researchers started by investigating the single-cell gene expression changes in the Alzheimer’s brain. They focused on both the neurons and glial cells, identifying the confusing bits of gene regulation altered by the disease.
- Database Comparison: By comparing the Alzheimer’s disease signature with expression profiles of 1,300 FDA-approved drugs, the team was able to determine which medications had the potential to reverse these changes. This stage involved sorting out fine details that required rigorous computational analysis.
- Medical Records Analysis: Millions of electronic health records were explored to compare the incidence of Alzheimer’s in patients who had previously taken some of these drugs. This step was critical, as it provided real-world evidence beyond the laboratory setting.
- Preclinical Testing: Finally, the top two candidate drugs, both originally developed for cancer treatment, were tested in a mouse model of Alzheimer’s disease. The results were highly encouraging, with the treated mice showing reduced brain degeneration and improved memory functions.
Table: Summary of the Research Process
| Research Step | Description |
|---|---|
| Gene Expression Analysis | Identification of Alzheimer’s-related changes in neurons and glial cells. |
| Drug Profile Comparison | Comparison of 1,300 FDA-approved drugs to find those that reverse gene changes. |
| Real-World Data Analysis | Examination of electronic medical records to observe Alzheimer’s incidence in drug takers. |
| Preclinical Testing | Evaluation of drug combinations in a mouse model for their effect on cognitive function. |
Turning Complex Data into a New Treatment Strategy
The use of computational tools to untangle the issues of Alzheimer’s has been both innovative and essential. By employing advanced algorithms, scientists could dig into vast amounts of data that would otherwise be overwhelming. This process did more than merely identify potential drug candidates; it offered a fresh perspective on how intertwined conditions like cancer and dementia can share similar, albeit complicated, biological features.
One of the key insights was the realization that the gene expression changes seen in Alzheimer’s are not random but follow a specific pattern. The drugs identified in the study appear to work by counteracting this pattern, essentially “resetting” the affected cells. This sort of computational health science has enormous potential, as it may pave the way for future breakthroughs in treating a wide range of conditions that are loaded with confusing bits of cellular alterations.
Potential Benefits and Broader Implications for Patient Care
From a patient care perspective, the repurposing of cancer drugs for dementia treatment brings a number of super important advantages. First, using medications that are already approved by regulatory authorities like the FDA could significantly speed up the time it takes to bring a new Alzheimer’s treatment to market. Second, because these drugs have documented safety profiles and known side effects, clinicians can have more confidence when considering them for new therapeutic applications. Lastly, this cross-therapy approach might inspire further research into other diseases that share similar gene expression disturbances or tangled issues between seemingly disparate medical fields.
For patients and caregivers facing the relentless decline associated with Alzheimer’s, such innovative therapies are welcome news. However, it is also crucial to acknowledge that repurposing cancer medications is not a silver bullet. The potential risks and side effects, especially when dealing with a delicate organ like the brain, must be managed carefully. The approach calls for robust clinical trials to determine the appropriate dosage and combination, ensuring that the benefits outweigh the risks.
Challenges in Repurposing Cancer Medications for Alzheimer’s
Integrating cancer treatments into the realm of dementia care is not without its intimidating challenges. Although the preliminary results in mouse models are promising, human biology is much more complex, and the path to a widely approved treatment is full of potential hurdles. Here are some of the tricky parts of repurposing these medications:
- Safety Concerns: Cancer treatments often come with a range of side effects. While the doses used for dementia might be lower, there is still a risk of adverse reactions, especially in the elderly population.
- Dosing and Administration: The dosage that works in a mouse model might not translate directly to humans. Identifying the correct dosage that is both safe and effective is a nerve-racking aspect of this research.
- Long-Term Effects: The potential long-term consequences of using cancer drugs in a new context remain on edge. Continuous monitoring and extended clinical trials are essential to ensure patient safety over time.
- Regulatory Hurdles: Even though the drugs are already FDA-approved, repurposing them for a new indication means navigating a completely different regulatory pathway. This can be a somewhat intimidating process for drug manufacturers and researchers alike.
Table: Potential Challenges in Repurposing Cancer Medications
| Challenge | Description |
|---|---|
| Safety Concerns | Managing side effects and adverse reactions in a non-cancer population. |
| Dosing Strategy | Identifying the correct dose that is both effective and safe in the elderly. |
| Long-Term Monitoring | Ensuring collection of sufficient long-term data to observe unforeseen consequences. |
| Regulatory Approval | Meeting the criteria for a new indication under a different set of regulations. |
Understanding the Statistical Evidence Behind the Findings
The study’s integration of statistical methods elevated its conclusions beyond theoretical possibility. By examining millions of electronic medical records, the researchers could get around the limitations of lab-based analysis alone. This real-world evidence suggested that patients who had taken some of the candidate drugs for other conditions were statistically less likely to develop Alzheimer’s disease. Although statistical analyses in healthcare can be loaded with problems, the careful design of this study and the sheer volume of data analyzed provided a persuasive argument in favor of the new approach.
This statistical insight not only supports the potential efficacy of the drug combination but also underscores the importance of using data in a systematic manner. It serves as a reminder that methodically analyzing massive datasets, even when faced with nerve-racking twists and turns, can reveal subtle details that might have been missed in a smaller study.
Computational Health Sciences: Paving the Way for Future Discoveries
One of the most exciting aspects of this research is its reliance on computational health sciences. As medicine becomes increasingly digital, the ability to poke around massive data sets has opened up new opportunities for drug discovery. Computational methods allow researchers to sift through thousands of potential candidates quickly, identifying which drugs are most likely to have an effect on complicated disease presentations such as Alzheimer’s.
By applying sophisticated algorithms and drawing upon global databases, scientists are now in a position to connect the dots between diseases that once seemed completely unrelated. This cross-disciplinary strategy is super important in modern medicine, where the most successful treatments often emerge at the intersection of different fields. The success of these computational tools in this study could encourage further efforts to identify repurposable drugs for a variety of conditions that have been, until now, considered too tangled in their gene expression changes to treat with traditional methods.
Potential Implications for the Future of Dementia Treatment
The early success of using these cancer medications in a mouse model is a sign of hope for the millions who battle Alzheimer’s disease. There is genuine optimism that by steering through the complex facets of gene expression and repurposing existing drugs, researchers might offer treatments that are not only effective but also accessible sooner than a brand-new drug might be. Here are some of the possible future implications:
- Accelerated Clinical Trials: With two of the drugs already being FDA-approved, clinical trials aimed at establishing their efficacy in Alzheimer’s patients could move forward more rapidly.
- Cost-Effective Treatments: Repurposing drugs often costs less than developing new treatments from scratch. This is a big plus for both patients and healthcare providers.
- Refinement of Treatment Strategies: A combined therapeutic approach that targets both the inflammatory and degenerative components of Alzheimer’s might offer a more balanced treatment plan, one that considers the nerve-racking challenges of the disease’s progression.
- Impact on Overall Healthcare: If the repurposing strategy proves successful, it could set a precedent for using similar computational and cross-disciplinary techniques to treat other neurological or age-related conditions.
Nonetheless, these possibilities must be weighed against the side effects and risks involved. It remains essential that any future treatment set-up undergo rigorous clinical evaluation to account for all the hidden complexities inherent in human biology. Even with the promise of these findings, both the delicate nature of the brain and the vulnerability of patients dealing with dementia call for caution and comprehensive testing.
The Role of Regulatory Authorities and Public Health Policy
Another significant factor in translating these findings from the lab to the clinic is the involvement of regulatory authorities such as the Food and Drug Administration. The dual role of ensuring safety and accelerating innovation is a balancing act that sometimes feels like steering through a maze of intertwined regulations. On one hand, the familiarity with approved dosages can make the process faster, but on the other, the shift in the therapeutic indication presents a set of nerve-racking challenges.
Public health policies must adapt to support innovative approaches that combine treatments from different fields. As research continues, it will be important for policymakers to consider incentives and streamlined regulations that allow scientifically backed repurposing methods to reach patients quickly, while ensuring that no safety steps are skipped. With the current pace of research in computational health sciences, the likelihood of similar breakthroughs in other areas is high, which could transform how we handle diseases historically burdened by limited treatment options.
Interdisciplinary Collaboration: The Future of Medical Innovations
The success of this study is a testament to the importance of interdisciplinary collaboration in medicine. On one side, oncologists bring in their expertise on cancer medications, a field loaded with decades of research and clinical experience. On the other, neurologists and computational scientists provide insights into the brain’s delicate and complicated pieces. This kind of teamwork is essential when tackling a disease as multidimensional as Alzheimer’s.
Collaboration across these fields allows researchers to make informed decisions based on a variety of perspectives. For instance, the decision to utilize computational tools was influenced by insights from data science experts who have experience in analyzing big, real-world datasets. At the same time, clinicians contribute an understanding of patient care and the subtle details that should be monitored during treatment. Combining these areas of expertise leads to more robust research designs and ultimately, innovative solutions that can benefit patients on multiple levels.
Table: Benefits of Interdisciplinary Collaboration in Drug Repurposing
| Discipline | Contribution |
|---|---|
| Oncology | Deep knowledge of drug mechanisms and safety profiles of cancer medications. |
| Neurology | Expert insights into brain function, neuron health, and dementia mechanisms. |
| Computational Sciences | Ability to analyze large datasets and identify subtle twists in gene expression patterns. |
| Clinical Research | Experience in designing and managing trials to affirm safety and efficacy in patients. |
Exploring the Small Distinctions in Gene Expression Outcomes
The study’s remarkable achievement lies in its detailed examination of the small distinctions—or fine shades—observed in gene expression among different brain cell types. Alzheimer’s disease introduces twists and turns in gene regulation, affecting neurons, astrocytes, and other supportive cells in ways that can seem both subtle and significant. By focusing on reversing these specific changes rather than treating the disease in a broad manner, researchers are taking the wheel in a much more targeted approach.
This strategy reflects a larger trend in medicine where treatments are moving from generalized therapies toward more personalized interventions. Instead of one-size-fits-all solutions, the goal now is to tailor treatment plans based on the individual genetic and molecular profiles of patients. This not only enhances the effectiveness of therapies but also minimizes unintended side effects, making treatment more patient-centered and ultimately more sustainable in the long run.
Real-World Impact: Stories and Statistical Trends
When looking at the potential impact of repurposing cancer drugs for Alzheimer’s, it is important to consider both the statistical trends and the personal stories of patients and their caregivers. While the study demonstrates encouraging statistical correlations between drug usage and reduced Alzheimer’s incidence, the real-world narrative is equally compelling.
Many families face the nerve-racking reality of watching a loved one gradually lose precious memories. For these individuals, even a modest improvement in cognitive function could translate into a dramatically better quality of life. Although the research is still in the early stages, the fact that a treatment may be derived from an existing drug offers hope—hope that someday, the overwhelming burden of dementia can be lessened through a strategic, data-driven approach.
- Case Studies: Preliminary case studies have shown that patients who incidentally received one or both of these cancer medications for unrelated conditions displayed fewer signs of cognitive decline.
- Healthcare Cost Implications: If these medications prove effective, the increased adoption could potentially reduce long-term healthcare expenses associated with advanced dementia care.
- Quality of Life Improvements: Even small enhancements in memory and cognitive function can help reduce caregiver stress and improve daily living activities for patients.
Charting a Future Filled with Possibilities
Looking ahead, the merging of oncology and neurology through computational health sciences is likely to yield even more extraordinary discoveries. In many ways, this study marks the beginning of a new era—an era where drugs are no longer confined by the conditions for which they were originally developed. Rather, the innovative use of existing treatments could open the door to a host of novel therapies that address multiple conditions concurrently.
As researchers continue to test and refine these methods, we can expect to see further advances that build on the initial success of this study. It is important, however, to remain mindful of the nerve-racking twists and turns that characterize early-stage research. Each new finding must be carefully validated through controlled clinical trials and supported by both statistical evidence and clinical experience.
Managing the Road Ahead: Clinical Trials, Safety, and Evolving Guidelines
One of the primary challenges moving forward is the design of clinical trials that can adequately capture both the benefits and the potential risks of repurposing cancer medications for dementia. These trials must take into account not only the immediate effects on cognitive function but also the long-term safety of these drugs when used in a new therapeutic context.
For example, researchers will need to monitor patients closely for any adverse effects that might not have been apparent in the shorter mouse model studies. The timing and dosage of administration could vary widely from those used in traditional oncology, which means that healthcare providers will have to figure a path through a carefully monitored treatment strategy. This aspect of clinical research is both critical and challenging, given the delicate balance between treatment efficacy and patient safety.
- Trial Design Considerations:
- Appropriate dosing regimens that differ from oncology use.
- Extended treatment durations to monitor long-term effects.
- Robust monitoring protocols for adverse events.
- Regulatory Compliance:
- Adapting existing FDA guidelines to new therapeutic applications.
- Maintaining transparency in reporting trial outcomes.
- Patient Selection Criteria:
- Identifying patients who may benefit the most based on genetic markers.
- Ensuring a diverse study population to capture subtle differences across demographics.
Guidelines for clinical trials will also need to evolve as data from ongoing studies become available. It is likely that we will see interim analyses and adaptive trial designs that allow researchers to make adjustments on the fly—a process that, while sometimes overwhelming, is essential for ensuring the best possible outcomes in a field riddled with tension and full of problems.
Reflections on the Broader Implications
The potential of repurposing cancer medications for the treatment of Alzheimer’s extends far beyond a single therapeutic breakthrough. It challenges long-standing perceptions about the rigid boundaries that separate different areas of treatment and opens up exciting possibilities for interdisciplinary medical practice. Through this work, we are reminded that innovation often comes from taking a closer look at the small distinctions in our understanding of disease—not simply from overall symptom management.
Moreover, this approach encourages a rethinking of how we view the drug development pipeline. Traditional methods can sometimes be off-putting in their pace and expense. By contrast, repurposing approved medications can offer a shortcut to effective treatments—albeit one that must be approached with careful thought and methodical testing. This model of innovation could serve as a blueprint for tackling other conditions where traditional research has reached an impasse.
Concluding Perspectives: Steering Through a Future of Hope
In the intricate and often nerve-racking world of medical research, breakthroughs like the repurposing of cancer medications for Alzheimer’s provide a much-needed beacon of hope. By working through the tangled issues of gene expression and leveraging the power of computational tools, researchers are offering a glimpse into a future where conditions that have long been viewed as intractable may finally be brought under control.
While there are still many questions to be answered, the potential implications of these findings are both promising and transformative. It is a reminder that sometimes, the key to solving one problem lies hidden in the tools developed to solve another. As scientists continue to dig into the data and steer through the complicated pieces of both oncology and neurology, patients and caregivers worldwide can look forward to treatments that are more targeted, effective, and, ultimately, life-changing.
In summary, the road ahead may be fraught with challenges, from the subtle details of drug dosing to the intimidating process of regulatory approval. Yet these obstacles also present opportunities for growth, collaboration, and innovation. The union of computational health sciences with traditional clinical research is paving the way for a new era where therapies are developed from a holistic understanding of human biology—a future that is as promising as it is exciting.
It remains essential for all stakeholders—researchers, clinicians, regulators, and patients—to stay engaged and informed as these pioneering studies progress into clinical practice. Through persistent collaboration and an open-minded approach to repurposing existing treatments, we may soon find ourselves on the brink of a revolution in how we understand and treat not just Alzheimer’s disease, but potentially many other complex conditions.
Ultimately, this breakthrough highlights the importance of exploring every available avenue in the fight against debilitating diseases. By harnessing the potential of cancer medications and applying them in innovative ways, we are not only discovering new treatment options for Alzheimer’s but also proving that the medical field is capable of surprising and transformative shifts. With continued research, collaboration, and determination, the blending of disciplines may well provide the keys to unlock cures for some of the most challenging health problems of our time.
As we take a closer look at these developments, it becomes clear that the future of medicine is likely to be defined by interdisciplinary approaches and creative solutions. The road may be tricky and filled with tangled issues, but every small twist and turn brings us closer to a world where diseases once thought unbeatable can be managed—and even overcome—by repurposing the tools already at our disposal.
This opinion piece is a call to action for the entire medical community: to remain curious, to always dig into the data, and to keep an open mind about how treatments from one field might hold the solution to another. In a landscape that is as dynamic as it is challenging, such innovative thinking is not just welcome—it is absolutely necessary for the advancement of global healthcare.
We now stand at the intersection of oncology and neurology, driven by computational advances and a deep desire to improve patient care. As research continues and clinical trials move forward, this promising approach may ultimately redefine how we think about and treat dementia, proving that sometimes, hope comes from the most unexpected of places.
Originally Post From https://oncodaily.com/insight/e-shyam-p-reddy-334884
Read more about this topic at
Cookie Consent | Products
Cookie Consent For GDPR & CCPA Compliance