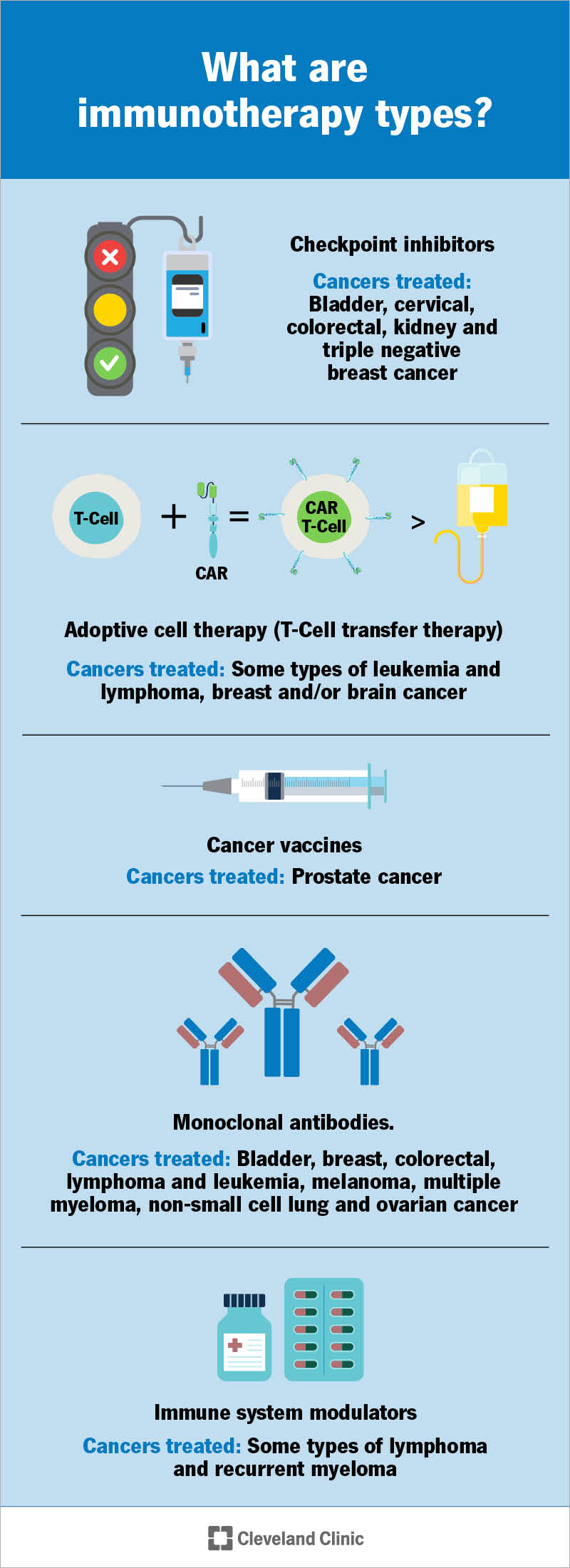
Revolutionizing Cancer Immunotherapy Through Molecular Insights
Recent breakthroughs in biomedical research are opening up fresh avenues in the battle against cancer. A new study has illuminated a molecular brake, known as STUB1, which appears to limit the natural cancer-fighting power of T cells. This discovery not only challenges our current understanding of immunotherapy but also intensifies the prospect of developing next-generation treatment strategies. In this opinion editorial, we take a closer look at these advancements, discuss their potential impact on future therapies, and explore both the promising prospects and the nerve-racking uncertainties that remain.
From the outset, it is clear that the role of the immune system in combating cancer is far more nuanced than it had been assumed. The research highlights the tricky parts of T-cell function and suggests that by tweaking the genetic makeup of these cells, we can potentially coax them into being more aggressive in their attack on tumor cells. As the field of cancer immunotherapy continues to evolve, the insights generated by this study may be the catalyst needed to overcome some of the tangled issues that have long plagued current treatment modalities.
Understanding the Tricky Parts of T-Cell Function
T cells, particularly the CD8+ subset, are already recognized as the immune system’s elite fighters. Yet, as this research demonstrates, there are complicated pieces within these cells that limit their effectiveness. STUB1, a protein that functions as a molecular brake, hinders their ability to recognize and attack cancerous cells. While current immunotherapies have transformed cancer treatment, they are effective in less than half of the patient population. This leaves a significant number of people without the potential life-changing benefits of these treatments.
The Role of STUB1: A Molecular Brake on Immunity
At the heart of this study lies the discovery of STUB1’s inhibitory effects on T-cell activity. By interfering with the receptors that are central to immune cell activation, STUB1 essentially dials down the war cry of the immune system. In experimental models, researchers found that when STUB1 is removed or disabled, T cells become markedly better at combating tumors. The genetic reprogramming that occurs without STUB1 results in a more vigorous and responsive T-cell population, one that is better equipped to detect and destroy malignant cells. This concept is not just a technical correction but a potential overhaul of our approach to immunotherapy.
How Gene Editing Has Changed the Game
The research team employed the gene-editing technology CRISPR to screen nearly 900 genes in search of those that might be preventing T cells from mounting an effective attack on cancer. In this process, STUB1 emerged as a standout candidate whose deletion markedly enhanced the T cells’ tumor-killing capabilities. The use of CRISPR allowed scientists to poke around in the genetic makeup of these cells, identifying hidden complex parts that were previously overlooked.
Below is a summary table that outlines the key steps from the research process:
| Step | Description |
|---|---|
| Gene Screening | Nearly 900 genes were evaluated using CRISPR to pinpoint those affecting T-cell function. |
| Identification | STUB1 was identified as a protein that restrains the anti-tumor response of CD8+ T cells. |
| Validation | Removing STUB1 from T cells resulted in improved tumor attack in mouse models. |
Gene editing has thus emerged as an essential tool for navigating the tangled issues within T-cell biology, paving the way for innovative treatment approaches that extend beyond traditional immunotherapies.
Diving Into the Confusing Bits of Cytokine Signaling
While the discovery of STUB1’s role offers significant enthusiasm, it also brings to light the confusing bits of cytokine signaling—a process integral to T-cell activation. Cytokines are essential signaling molecules that instruct the immune cells on when to strike. One cytokine in particular, IL-27, plays a key role in this process. By blocking STUB1, researchers observed that T cells exhibit an enhanced response to IL-27, suggesting that the interplay between STUB1 and cytokine receptors is more critical than previously appreciated.
IL-27: The Underestimated Immune Messenger
IL-27 has not traditionally been in the limelight when it comes to cancer immunotherapy. However, the new findings indicate that it holds a key, though often overlooked, role in tuning the immune response. Under normal circumstances, T cells depend on IL-27 signaling to mount an effective attack on tumor cells. When STUB1 slows down this signaling mechanism, the cells are left somewhat handicapped in their fight. The potential to boost IL-27 activity by inhibiting STUB1 opens up a promising strategy to supercharge the immune response—a discovery that could lead to therapies benefiting a wider range of patients.
Disentangling the Signal Interactions: A Closer Look
Understanding how STUB1 interferes with cytokine receptors involves getting into a level of detail that can be quite overwhelming. In essence, STUB1 works by interacting with another protein called CHIC2. This interaction results in the removal of key receptors from the surface of T cells, thus blunting their responsiveness to immune-activating signals. To better illustrate the process, consider the following bullet list of the primary steps:
- T cells rely on cytokine receptors to receive activation signals.
- STUB1 partners with CHIC2 to remove these receptors from the cell surface.
- With fewer receptors available, the T cells’ ability to respond to IL-27 and other signalling molecules is reduced.
- The overall anti-tumor response is therefore subdued, allowing tumor cells to thrive.
These subtle parts of cell signaling highlight how fine shades in the immune response can greatly influence the outcome of cancer therapies. The research suggests that by disabling the STUB1-CHIC2 interaction, we can preserve the necessary receptors on T cells, enabling them to receive a full signal from cytokines like IL-27.
Exploring the Nuanced Challenges in Enhancing Cancer Immunity
The clinical implications of these findings are profound, yet they are not without significant challenges. While boosting T-cell function by targeting STUB1 appears promising in laboratory models, translating this approach to human patients is loaded with issues and potential pitfalls. The results obtained in mouse models must be interpreted cautiously before they are applied in clinical settings.
Among the challenges, the possibility of off-target effects and the overall safety profile of such interventions remain of key concern. The removal of a protein as broadly expressed as STUB1 could have unintended consequences, including immune-related toxicities. However, the research points to a solution: targeting STUB1 selectively in cancer-fighting T cells might help mitigate these risks, creating a more focused approach that minimizes systemic side effects.
Potential Risks and Rewards in Tumor Vulnerability
It is essential to weigh the benefits of enhanced T-cell activity against the potential risks associated with the manipulation of immune functions. On the rewarding side, engineered T cells that lack STUB1 have demonstrated slower tumor growth and longer survival in preclinical studies. They also present a dual advantage. Not only do T cells become more aggressive in attacking tumors, but the tumors themselves may become more vulnerable to immune cell infiltration. In theory, this could lead to a compounded therapeutic effect.
That said, the clinical translation of such strategies is not without nerve-racking challenges. The journey from mouse models to human applications is full of tricky parts, and the safety of long-term STUB1 inhibition must be established through rigorous clinical trials. Researchers are now faced with the off-putting task of designing strategies that can both enhance T-cell function and limit unintended systemic impacts.
Engineering T Cells: A Promising Future
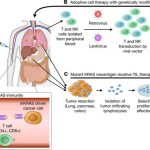
One of the most exciting prospects emerging from this research is the possibility of re-engineering patients’ own T cells to remove or inhibit STUB1. This cell-based approach is seen as a promising frontier in cancer treatment. By modifying T cells ex vivo, clinicians could potentially deliver a highly efficient version of the immune cell that is primed to attack cancer without the drawbacks of broader systemic inhibition.
The process of engineering T cells involves isolating the cells from the patient, editing the genes to remove or downregulate STUB1, and then reintroducing them back into the patient’s body. This method provides a controlled environment to manage the changes and monitor the cells for any adverse reactions before they are deployed into the body’s complex landscape.
Below is an outline of the potential steps involved in a T-cell engineering process:
- Isolate T cells from the patient’s blood sample.
- Utilize CRISPR or similar gene-editing techniques to target and disable STUB1.
- Validate the modified T cells in a controlled laboratory setting to ensure enhanced receptor expression.
- Expand the population of engineered T cells before reintroducing them into the patient.
- Monitor patient response and adjust the therapeutic strategy as necessary.
This targeted approach could potentially overcome many of the current obstacles in cancer immunotherapy by providing a more direct, cell-based solution that exploits the body’s natural defense mechanisms.
Making Your Way Through New Treatment Possibilities
The integration of this research into clinical practice could mark a turning point in our battle against cancer. For patients who do not respond to current immunotherapeutic regimens, the inhibition of STUB1 might offer an alternative strategy. As scientists make their way through these innovative treatment possibilities, they must carefully balance the enhancement of the immune response with the need to avoid unintended side effects.
A multifaceted approach combining STUB1 inhibition with existing therapies might prove to be the most effective method. Researchers are exploring whether augmenting T-cell priming via STUB1 blockade could be synergistic with immune checkpoint inhibitors or other immunomodulatory drugs that bolster the immune response at later stages of action. With the potential for combination therapies, the next phase of research is likely to involve rigorous preclinical and clinical studies to map out the best treatment protocols.
Combining Therapies for Enhanced Immune Response
One promising strategy involves pairing the STUB1-targeted approach with conventional immunotherapies. For example, checkpoint inhibitors that release the brakes on T cells’ later-stage responses might work in tandem with early-stage enhancements provided by the removal of STUB1. Such a dual strategy could lead to a more comprehensive activation of the immune system against tumors.
- Checkpoint Inhibitors: These drugs remove inhibitory signals in T cells, allowing them to attack cancer cells more vigorously.
- STUB1 Inhibition: By preventing the loss of cytokine receptors, this strategy ensures that T cells can fully respond to immune signaling molecules.
- Combined Therapy: Using both strategies simultaneously might yield a potent synergistic effect, optimizing the entire T-cell response.
Such combination therapies not only promise a stronger anti-tumor response but also add layers of safety by relying on multiple mechanisms, thereby reducing the risk that comes from tweaking just one aspect of a highly complex immune system.
Addressing the Overwhelming and Nerve-Racking Concerns
Even as we embrace these promising advances, it is necessary to acknowledge the intimidating challenges that lie ahead. Transitioning from the lab bench to the patient bedside is, without a doubt, nerve-racking and full of potential setbacks. There are practical issues to consider: the effectiveness of engineered T cells in the diverse human body, the stability of gene-edited cells over time, and the possibility of triggering adverse immune reactions.
Moreover, the translation of findings from mice to humans is a journey dotted with twists and turns. What works in controlled laboratory environments sometimes encounters unexpected barriers when applied to human physiology. Nevertheless, through careful research and measured progress, these concerns can be gradually addressed, paving the way for therapies that are both innovative and safe.
Long-Term Implications for Cancer Treatment Innovations
Looking well beyond the horizon of immediate clinical applications, the current research carries significant long-term implications. By uncovering one of the fine points of immune regulation—the STUB1 pathway—scientists are setting the stage for a new era where cancer treatments might be tailored with far greater precision. Even though the initial studies have been conducted in animal models, the consistency of the findings in human cell experiments adds optimism to the translation of these techniques into future patient care.
The discovery that targeting STUB1 can both rev up T-cell responses and increase tumor vulnerability represents a dual-pronged attack on cancer. This might be especially critical for patients who have not responded well to existing immunotherapies. By essentially re-engineering the immune ecosystem, researchers are opening up a landscape filled with potential. Yet, the actual clinical success of such innovations will depend on our ability to solve the little twists and unexpected difficulties that always arise when experimenting with the body’s complex systems.
Benefits of the Discovery for Future Treatments
- Enhanced T-Cell Activation: The removal of STUB1 boosts the T cells’ ability to detect and kill cancer cells, leading to improved anti-tumor activity.
- Combination Therapy Success: When used alongside existing treatments such as checkpoint inhibitors, STUB1 inhibition might lead to more robust and comprehensive immune responses.
- Precision Medicine Approach: Engineering a patient’s own T cells to disable STUB1 could offer a more tailored treatment, reducing the risk of systemic side effects.
- New Therapeutic Targets: The study opens avenues to explore other hidden proteins and pathways that affect T-cell performance, broadening the horizon for future research.
Potential Hurdles in Clinical Applications
Despite the clear benefits, there are several points of caution that researchers and clinicians must address before moving forward with STUB1-targeted therapies. These include:
- Safety Concerns: Given that STUB1 is widely expressed in different cell types, systemic inhibition may lead to unanticipated toxicities. Careful modulation that is limited to T cells is crucial.
- Long-Term Effects: The stability and long-term behavior of gene-edited T cells in the human body remain to be fully understood.
- Translational Gaps: The step from successful mouse experiments to human treatments is full of tricky parts that require extensive clinical testing.
- Regulatory Challenges: Gene-editing and cell-based therapies are subject to rigorous regulatory scrutiny, which can slow down the translation to clinical practice.
Conclusion: A New Chapter in Cancer Immunotherapy
In conclusion, the discovery of STUB1’s role as a molecular brake in T-cell immunotherapy is both promising and provocative. It offers a fresh perspective on how we can work through the tangled issues of immune signaling to create more effective cancer therapies. By removing or disabling STUB1, researchers are not only enhancing the T cells’ ability to detect tumors but are also potentially rendering cancer cells more vulnerable to immune attacks.
The potential of this breakthrough extends beyond immediate clinical applications. It prompts us to reimagine cancer treatment strategies by moving away from one-size-fits-all solutions and toward more personalized, cell-based approaches. Although the road ahead is loaded with problems and nerve-racking challenges, the opportunity to revolutionize cancer care is too significant to ignore.
As we cautiously dig into these research findings, it becomes clear that the future of cancer treatment may well depend on our ability to manipulate the immune system at its most subtle levels. The ingenuity of using CRISPR to sift through nearly a thousand genes and isolate STUB1 highlights the power of modern genetic tools in exposing the hidden complexities underlying our body’s defenses.
For clinicians, researchers, and patients alike, this development is a reminder that medical innovation is an evolving journey. With every new discovery, we find our way through a maze of small distinctions and intricate interactions that can ultimately lead us to safer and more effective treatments. While the translation of these findings into widespread clinical practice will require further studies, rigorous testing, and regulatory approvals, the study offers a hopeful glimpse into what could be the next big leap forward in cancer immunotherapy.
The idea of combining STUB1 inhibition with existing therapies paves the way for innovative treatment combinations that might offer improved outcomes. Each step forward, no matter how nerve-racking or intimidating it may seem, represents progress in our ongoing fight against cancer.
In the grand scheme of medical progress, it is the drive to understand and optimize every subtle detail that truly propels us forward. There is a real potential for the blocking of STUB1 to act as a standalone therapy or be used in conjunction with other immunotherapies, thereby opening up a dual-front attack on tumor cells. This multifaceted approach not only reinforces the body’s natural defenses but also exemplifies the spirit of innovation that has long defined modern medicine.
As with any transformative discovery, the conversation around STUB1 and its role in modulating the immune response is poised to stimulate vigorous debate among oncologists, immunologists, and molecular biologists. It is essential for all stakeholders—researchers, clinicians, and policymakers—to work together to pave the way for safe and effective clinical applications.
Ultimately, while the path ahead is undoubtedly full of challenges and tricky parts, the potential benefits of harnessing the immune system to fight cancer cannot be overstated. The magic of science lies in taking these nerve-racking, off-putting uncertainties and transforming them into life-saving innovations. With measured progress, careful evaluation, and a willingness to take calculated risks, the next chapter in cancer immunotherapy might be just around the corner.
The progress presented by this study calls on us to continue probing the intricate balance between our immune defenses and the stealthy strategies employed by cancer cells. It reminds us that even in the face of overwhelming challenges, the pursuit of scientific understanding and innovation can lead to breakthroughs that shape the future of medicine.
In summary, while we must respectfully acknowledge the many small distinctions and subtle points at play, we are nonetheless witnessing the emergence of a promising strategy in cancer treatment. The insights gained by disabling STUB1 not only deepen our understanding of the immune system’s inner workings but may also herald a new era in which more patients can benefit from advanced, tailored therapies.
It is incumbent upon the medical community to support and accelerate this line of research. With further studies—alongside a careful assessment of clinical safety and efficacy—the hope is that therapies targeting STUB1 and its associated pathways will soon transition from the lab bench to the patient’s bedside. For those in the fight against cancer, these developments offer a beacon of hope, lighting the way through a landscape that is as promising as it is challenging.
Originally Post From https://hms.harvard.edu/news/research-identifies-new-ways-supercharge-cancer-immunotherapy
Read more about this topic at
Unlocking antitumor immunity with adenosine receptor …
Unlock the Code of MHC II-enabled Cancer Immunotherapy