Finding Your Path in Oncology: The Rise of Biosimilars and 505(b)(2) Drugs
In today’s rapidly evolving oncology landscape, a growing number of treatment strategies are shifting the focus toward cost-effective alternatives. Biosimilars and 505(b)(2) drugs have captured the attention of healthcare professionals by offering promising options beyond traditional biologics and brand-name medications. However, the introduction of these drugs brings with it a host of tricky parts and tangled issues that demand careful thought from everyone involved—from clinicians to pharmacists and payers.
This editorial explores the operational, clinical, and policy strategies that healthcare professionals must consider to make these therapies work efficiently in cancer care. With an objective look at the confusing bits, we will get into how pharmacists, in particular, play a key role in steering through these emerging treatment options while ensuring patients receive both quality care and financial relief.
Operational Challenges and Workable Strategies
Biosimilars and 505(b)(2) drugs are frequently touted for their potential to reduce costs; they are typically less expensive than their originator counterparts. Yet, beneath the promising headlines lie several tricky operational challenges that need to be managed effectively. The successful rollout of these therapies requires close teamwork between pharmacists, payers, and providers.
Up-Front Substitution and the Start-of-Treatment Advantage
One prominent operational strategy endorsed by experts in oncology involves making a substitution at the very start of the treatment regimen. In this model, biosimilars are introduced as early as possible, minimizing workflow disruptions later on. Many experts agree that beginning treatment with a preferred alternative reduces the administrative burden that often accompanies mid-treatment switches. This approach not only smoothes out the transition but also lessens the overwhelming nature of changing medications once a treatment plan is already in progress.
Key points for implementing up-front substitution include:
- Starting with a preferred biosimilar during the initial treatment phase
- Reducing the need to re-document or reauthorize treatments mid-course
- Improving clinical continuity through streamlined protocols
Healthcare institutions are now adopting policies that reinforce the importance of such early substitution, ensuring that both clinical practice and administrative processes align. In contrast, while commercial payers might offer more flexibility, Medicare regulations remain more conservative by restricting step therapy involving biosimilars to new treatment episodes.
Medically Integrated Dispensing: A Hands-On Model
Medically integrated dispensing (MID) is gaining traction as a best-practice model for managing biosimilars and 505(b)(2) drugs. In a MID system, pharmacy services are tightly woven into the clinical fabric of oncology clinics. This hands-on approach ensures that pharmacists are not just dispensing medications but are actively involved in building EHR order sets, educating providers, and managing inventories in real time.
The benefits of MID include:
- Real-time alignment of treatment protocols with payer preferences
- Proactive educational outreach to both providers and patients about therapy options
- Streamlined management of supply-related issues with fewer confusing bits affecting timely care
This model provides an effective counterbalance to the needle-in-a-haystack task of integrating new therapies, ensuring that each step is coordinated and that the process remains transparent and manageable despite its twists and turns.
Clinical Considerations: Steering Through the Fine Points of Therapy Integration
The clinical integration of biosimilars and 505(b)(2) drugs presents its own set of tricky parts. On one hand, these medications can potentially transform cancer treatment through significant cost savings and improved accessibility. On the other, they introduce several complicated pieces—ranging from the need for distinct billing codes to variable reimbursement pathways—that must be kept in check.
Understanding the Subtle Differences Between Therapeutic Options
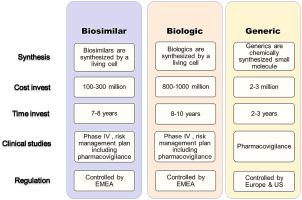
A critical part of managing these therapies is understanding that 505(b)(2) applications are not the same as generics. Whereas generics are usually therapeutically equivalent and can be substituted without hesitation, 505(b)(2) drugs rely heavily on prior clinical data and thus behave more like unique treatments with their own set of challenges.
Within the clinical realm:
- Clinicians need to be well informed about the fine shades separating biosimilars from 505(b)(2) drugs.
- For each 505(b)(2) product, understanding the billing and prior authorization requirements is critical to avoid nerve-racking delays in treatment.
- Knowledge about the underlying clinical data that supports these products is essential for reassuring both healthcare providers and patients.
By taking a closer look at these subtle parts, oncology teams can reduce the potential for clinical missteps and build confidence in the newer treatment options.
Patient Safety and Treatment Consistency
It is vital for the safety and well-being of patients that the integration of biosimilars and 505(b)(2) drugs does not lead to disruptions in their treatment regimen. The shift in therapeutic agents should be smooth and minimally invasive. For instance, consistent application by providers can be achieved by having well-established protocols that detail every step of the transition process.
Consider the following practices designed to preserve patient safety:
- Developing standardized treatment order sets within the EHR system to align with preferred therapies
- Implementing proactive monitoring systems that track clinical outcomes post-substitution
- Engaging in thorough documentation, especially when switching away from preferred products for clinical reasons
These measures help ensure the process remains on track, even when faced with potential bottlenecks and nerve-racking regulatory requirements.
Policy and Regulatory Considerations: Steering Through Administrative Labyrinths
Policy and regulatory environments shape the landscape in which biosimilars and 505(b)(2) drugs operate. While these treatments offer cost efficiencies and increased patient access, the administrative side of the equation is often full of problems and loaded with issues that require careful policy attention.
Collaboration Between Payers and Providers
One of the most effective strategies for managing the transition to these new therapies is robust collaboration between payers and healthcare providers. Payers can take proactive steps by evaluating prescribing patterns and aligning formularies with the most commonly prescribed products. This method not only helps reduce the overwhelming bits of paperwork associated with incorrect substitutions but also speeds up the authorization process.
Key considerations include:
- Evaluating provider prescribing patterns prior to the rollout of new therapies
- Selecting multiple preferred products to offer flexibility without compromising on consistency
- Ensuring that nonpreferred product usage is well-documented to authorize exceptions swiftly
These operational tactics show that aligning payer policies with on-the-ground clinical realities can lead to smoother implementation and a more positive impact on patient care.
Key Performance Indicators: Tracking Success Beyond Simple Utilization
While utilization rates provide a basic metric of adoption, deeper insights into the success of biosimilar and 505(b)(2) integration come from tracking additional operational indicators. These include:
| Metric | Description |
|---|---|
| Rate of Up-Front Changes | Frequency at which new treatment starts align with preferred therapies |
| Prior Authorization Denials | Incidences when nonpreferred product use results in delays or denials |
| Provider Responsiveness | The speed and consistency of providers in adapting to new formularies |
By tracking these nuanced operational measures, stakeholders can further refine their strategies, ensuring that each small twist in the implementation process is addressed promptly and efficiently.
Financial Navigation: Tackling the Cost and Reimbursement Maze
Costs and reimbursement considerations remain some of the more intimidating aspects of integrating biosimilars and 505(b)(2) drugs into oncology practice. Balancing cost-effectiveness without sacrificing quality requires that every healthcare professional—especially pharmacists—take an active role in financial navigation.
Understanding Patient Assistance Programs and Co-Pay Variations
In the realm of oncology therapies, patient assistance programs (PAPs) can vary widely between originator drugs, biosimilars, and 505(b)(2) therapies. Typically, originator biologics offer the most robust support, while biosimilars provide slightly more limited assistance. Meanwhile, 505(b)(2) drugs often fall into a category with minimal financial backing since they are already marketed as lower-cost alternatives.
Important points in this area are:
- Recognizing that differences in PAP availability can influence patient adherence to treatment
- Ensuring that financial navigation teams are aware of the specific eligibility criteria for each product
- Preparing to enroll patients in new support programs when switching from one therapy to another
Overcoming these financial twists and turns is critical because even minor delays in securing patient support can have a dramatic impact on treatment adherence and overall outcomes.
Strategies for Seamless Reimbursement Processes
Effective financial navigation means more than merely applying for assistance programs; it also involves ensuring that the reimbursement process is aligned with clinical policies. Pharmacists must collaborate with revenue cycle teams and work closely with insurance providers to minimize disruptions in care. Here are some strategies that have proven effective:
- Developing clear guidelines for documenting the clinical necessity of nonpreferred products
- Educating providers on the specifics of payer formularies to avoid unnecessary denials
- Building robust communication channels between clinical teams and billing departments to quickly handle reimbursement issues
Such proactive coordination can help guarantee that financial considerations do not stand in the way of timely patient treatment.
Cross-Team Coordination: Building Bridges Between Clinical and Administrative Functions
Perhaps one of the most essential strategies in this evolving landscape is the role of the pharmacist as a bridge between the clinical, administrative, and financial teams. With their unique expertise and close interaction with different facets of patient care, pharmacists are ideally situated to figure a path through the tangled issues of drug integration.
Breaking Down Silos for Better Communication
To ensure that biosimilars and 505(b)(2) drugs are implemented without a hitch, it is super important for different teams to work together. Pharmacists can take on the following roles:
- Learning the preferred therapies for each payer to ensure that the right drug is chosen from the start
- Collaborating with revenue cycle managers to monitor the progress of prior authorizations and reimbursements
- Providing vital education to clinical staff to help them understand subtle differences between therapeutic options
This collaborative effort helps reduce the nerve-racking delays that often stem from miscommunication. Instead of operating in silos, a cross-functional approach ensures that each step—from drug selection to the final dispensation—runs as seamlessly as possible.
The Role of Technology in Facilitating Coordination
Modern healthcare systems now rely heavily on technology to streamline workflows and support the communication between clinical and administrative teams. Electronic health records (EHR) and other integrated software solutions can be pivotal in managing formulary alignment and real-time inventory control. Pharmacists often use these tools to build treatment order sets that reflect payer preferences, deepening the connection between operational strategies and patient care.
By incorporating technology, teams can:
- Update treatment protocols in real time as new data and regulations emerge
- Ensure that all specialists—from oncologists to billing administrators—are on the same page
- Monitor the success of biosimilar and 505(b)(2) implementation through comprehensive dashboards
This hands-on approach, driven by modern data systems, ultimately helps teams steer through the confusing bits of complex regulatory and operational requirements.
Policy Evolution: Prospects for Therapeutic Interchangeability and Beyond
Multiple stakeholders view policy evolution as one of the key components for the wider adoption of biosimilars and 505(b)(2) therapies. The idea of making these alternatives automatically substitutable with their originator products appears as a dream scenario to many in the field—the kind of policy shift that would eliminate many of the complicated pieces currently faced by healthcare providers.
Therapeutic Interchangeability: A Game Changer?
Imagine a system where all biosimilars and 505(b)(2) drugs are deemed therapeutically interchangeable with their reference products. Such a change could streamline substitution practices, reduce the paperwork workload, and create a more competitive market that drives down costs. While current regulatory frameworks do not fully permit this level of interchangeability, progress in policy circles remains an optimistic prospect.
Key arguments in favor of therapeutic interchangeability include:
- Reducing administrative overhead, which is often loaded with issues
- Encouraging a more competitive market that can drive substantial cost savings for healthcare providers and patients alike
- Simplifying the process for pharmacists, thus allowing them to focus more on patient care rather than paperwork
Although achieving full interchangeability is a complex and sometimes scary uphill battle, it remains a pivotal goal for stakeholders seeking to make the oncology drug landscape more efficient and accessible.
Regulatory Frameworks: Balancing Innovation and Control
Regulators must strike a delicate balance between promoting innovative, cost-effective medication options and ensuring that any new therapies meet strict safety and efficacy standards. This balancing act is often full of problems and on edge discussions as policymakers work to address the confusing bits without compromising the integrity of treatment regimens. It is ultimately about protecting patients while encouraging progress in drug development.
Some of the fine points in the ongoing policy debate include:
- How to classify and regulate new 505(b)(2) products that don’t fit neatly into existing categories
- Determining when and how biosimilars may be safely substituted without a negative impact on clinical outcomes
- Ensuring that any regulatory changes are communicated clearly to avoid sudden workflow disruptions
The evolution of these regulatory frameworks is a process that will likely continue to present both challenges and opportunities for those navigating the oncology drug landscape.
The Road Ahead: Practical Insights for Oncology Professionals
As biosimilars and 505(b)(2) drugs increasingly become integral components of cancer treatment, oncology professionals stand at a critical juncture. The integration process is riddled with tension and challenges, but it is also loaded with opportunities for significant cost savings and improved patient access.
Empowering Pharmacists to Lead the Charge
Pharmacists are on the frontlines in managing the fine points of drug substitution and workflow management. Their expertise is super important not only in easing the administrative burden but also in ensuring that patients receive consistent, high-quality care. Experts in the field have demonstrated that through close collaboration with payers and providers, pharmacists can make a profound difference by:
- Taking the wheel in building practice-specific guidelines for drug substitution
- Leading cross-team trainings to ensure that the nuances of each therapy are understood and respected
- Using technology-driven tools to monitor and guide the transition process in real time
By empowering pharmacists, healthcare institutions can better align their treatment strategies with the evolving regulatory and clinical demands of modern oncology practice.
Building Sustainable Cost-Efficiency Models
Integrating cost-effective treatments like biosimilars and 505(b)(2) drugs is more than a clinical decision—it is a long-term strategy that requires well-organized financial planning. As institutions grapple with the various twists and turns of reimbursement and eligibility criteria, action plans that incorporate financial navigation and strategic payer collaboration can make all the difference in sustainable care delivery.
To build these models, healthcare teams must consider:
- Regular training sessions for financial navigation teams to keep up with evolving reimbursement policies
- Engaging in active partnerships with manufacturers to secure the best possible PAPs and co-pay support programs
- Maintaining transparent communication with patients regarding any changes in financial support as new therapies are introduced
These strategies help smooth out the nerve-racking processes associated with cost transitions and ensure that patient care remains consistent and accessible.
Conclusion: Steering Through the Future of Oncology Therapy
The journey toward integrating biosimilars and 505(b)(2) drugs into oncology care is a multifaceted endeavor—one that combines operational dexterity, clinical insight, and pragmatic policy adjustments. The road ahead will undoubtedly include tangled issues, overwhelming administrative bits, and nerve-racking challenges. Yet, with robust collaboration between pharmacists, payers, and providers, the oncology community is well poised to find its way through these tricky parts.
Through initiatives like up-front substitution, medically integrated dispensing, cross-team coordination, and proactive financial navigation, healthcare professionals are actively shaping a future where cost efficiency and high-quality patient care go hand in hand. As regulatory changes evolve and the market adapts to the unique needs of cancer care, the dream of full therapeutic interchangeability may one day transform this already dynamic landscape into a model of streamlined innovation.
Ultimately, the key takeaway is that every stakeholder—from the clinical practitioner to the administrative coordinator—plays a critical role in ensuring that these new therapies are adopted with minimal disruption. By working through the confusing bits of clinical guidelines and back-end operations, healthcare professionals can maintain the confidence of patients and ensure that oncology care continues to advance in a sustainable, patient-centered direction.
In our increasingly complex healthcare environment, the integration of biosimilars and 505(b)(2) drugs offers a beacon of hope—a promise of more accessible, cost-effective, and high-quality treatment for patients battling cancer. With informed decision-making, cross-functional teamwork, and active policy engagement, the future of oncology looks not only promising but also firmly in the hands of professionals committed to making a measurable difference in the lives of those they serve.
As the conversation continues and the landscape evolves, the oncology community must remain agile and open to new ideas. The twists and turns of this journey will require ongoing dialogue, careful evaluation of performance indicators, and a willingness to embrace change. Only then will the full benefit of these innovative therapies be realized—ensuring that each patient not only receives effective treatment but also experiences a smoother, less intimidating path through their healthcare journey.
In closing, while the shift toward biosimilars and 505(b)(2) drugs is loaded with challenges, it is also full of possibilities. Healthcare professionals must keep taking a closer look at every small distinction between therapeutic options, working through the operational and policy details, and maintaining patient-centric strategies at every turn. The road may be full of complicated pieces and nerve-racking administrative bits, but through persistent, collaborative effort, the oncology community can build a future where innovation and safety go hand in hand.
Originally Post From https://www.pharmacytimes.com/view/navigating-biosimilars-and-505-b-2-drugs-in-oncology-operational-clinical-and-policy-strategies-for-pharmacists
Read more about this topic at
Optimizing Biosimilar Implementation: A Framework for …
Biosimilars: Opportunities to Promote Optimization Through …