Innovative Breakthrough: Trojan Horse Bacteria and Oncolytic Viruses in Cancer Treatment
The latest research from Columbia University School of Engineering and Applied Science offers a fresh perspective on cancer treatment by combining bacteria and viruses into one cooperative system. In this opinion editorial, we take a closer look at this groundbreaking approach—one that uses bacteria as Trojan horses and oncolytic viruses as precision cancer-fighting agents. This new strategy not only promises to bypass the immune system’s tricky parts but also provides key safety mechanisms to ensure that the virus remains localized within the tumor site.
While many emerging cancer therapies face overwhelming challenges, this innovative method offers hope for many patients who have exhausted conventional treatments. It sparks both excitement and necessary debate across the oncological community, as experts get into the fine points of bacterial-viral synergy—exploring both its promising strengths and the tangled issues that still need clarification.
Understanding the Mechanism: Bacterial Trojan Horses in Oncology
This technology revolves around the concept of using bacteria that naturally seek out and invade low-oxygen, nutrient-rich environments, such as those found inside tumors. Once these specially engineered bacteria reach their target, they deliver a virus that is specifically tailored to attack cancer cells.
Bacteria as Covert Operatives
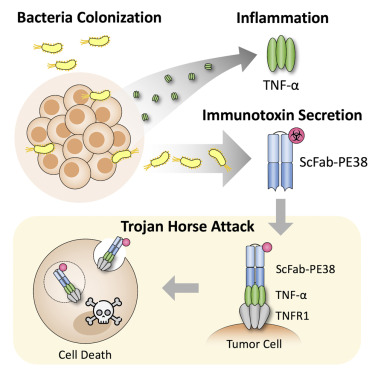
The bacteria used in this therapy, Salmonella typhimurium, have been modified to serve as safe carriers. Their natural tendency to find and settle within tumors is harnessed as they transport the virus directly to the malignant cells. Think of these bacteria as covert operatives making their way into enemy territory. They are strategically deployed to evade the body’s immune defenses—much like a well-planned infiltration mission.
By using the bacteria’s inherent tumor-seeking behavior, scientists have found a way to carry the virus past the body’s circulating antibodies. These antibodies can often neutralize therapeutic viruses if the patient has prior immunity. In this method, the bacterial “Trojan horses” safely hide their viral payload, allowing the virus to reach the tumor and then activate to destroy the cancer cells.
Key Points of the Bacterial Carrier System
- Targeting Efficiency: The bacteria naturally migrate toward areas with low oxygen and high nutrients, making them perfect for seeking tumors.
- Immune Evasion: By concealing the virus, the bacteria shield it from the patient’s immune system, bypassing the confusing bits related to immune neutralization.
- Direct Delivery: After homing in on the tumor, the bacteria self-destruct to release the virus, ensuring that the viral attack starts right where it is needed.
This approach cleverly overcomes some of the major hurdles associated with virus-only cancer therapies. The virus is protected from premature inactivation in the bloodstream, and once inside the tumor, it is free to replicate and target cancer cells.
Overcoming Immune System Hurdles: Evading Antibodies with Covert Delivery
When it comes to oncolytic virus therapy, one of the nerve-racking challenges has been the body’s own immune system. Many people have pre-existing antibodies, either from prior infections or vaccinations, which can neutralize the virus long before it reaches its target. The ingenious design of this bacterial-viral system circumvents this obstacle by tucking the virus safely inside bacteria.
How the Immune Shield Works
Once inside the bloodstream, traditional oncolytic viruses often face a barrage of antibodies prepared to attack and neutralize them. With the new system, however, the bacteria act as a protective cloak—an invisibility cloak, one might say—that enables the virus to evade these destructive antibodies. This strategy is especially important when dealing with viruses that many people have already encountered in their everyday lives.
By using bacteria to hide the virus, this method ensures that the viral payload remains intact until it is inside the tumor. Here, after the bacteria break down, the virus is released where it can spread among the tumor cells and exert its therapeutic effects. This is a key innovation in designing therapies that must outsmart our body’s own defensive mechanisms.
Table: Immune Evasion Comparison
| Traditional Oncolytic Therapy | Bacterial-Viral Hybrid Therapy |
|---|---|
| Virus directly exposed to immune system | Virus concealed inside tumor-seeking bacteria |
| Higher chance of antibody neutralization | Reduced risk of premature viral inactivation |
| Difficulty in directly targeting tumor cells | Efficient targeting using bacteria’s natural migration to tumors |
Targeting Cancer: Viral Payloads at Work Within Tumors
Once the bacterial carrier reaches the tumor, its primary mission is to release the viral payload. This virus then begins its work on infiltrating and destroying cancer cells. The concept is built on a dual-action strategy—leveraging both the virus’s natural ability to infect tumor cells and the bacteria’s knack for finding them.
How the Viral Attack Functions
Inside the tumor, the bacteria self-destruct, a phenomenon known as lysis, to release the engineered virus directly into the tumor’s interior. Once free, the virus spreads from one cancer cell to the next, replicating and causing cell death. This creates a self-sustaining cycle of infection and destruction, specifically targeted at the tumor.
This process has several significant advantages. For one, the virus is delivered right to its intended target, limiting collateral damage to healthy tissue. Moreover, because the virus is released only within the tumor microenvironment, it minimizes the risk of spreading to and harming healthy cells elsewhere in the body.
The Dual-Action Approach Explained
- Bacterial Homing Instinct: Salmonella typhimurium has an inherent ability to zero in on tumors due to the unique conditions found there, such as low oxygen levels.
- Intratumoral Virus Activation: The virus remains safely concealed until the bacteria release it upon reaching the tumor, ensuring its activation only where it is needed.
- Self-Limiting Viral Spread: The virus is designed so that it cannot replicate in areas outside the tumor, thanks to a molecular safeguard that ties its life cycle to the presence of the bacteria’s special protease.
This coordinated interplay between bacteria and virus paves the way for a more focused, effective, and safer cancer treatment strategy than many existing methods.
Built-In Safety Mechanisms: Ensuring Localized Action
One of the pivotal concerns in any live virus therapy is controlling the spread of the virus outside the target area. The research team tackled this head-on by engineering a system that prevents viral replication away from the tumor site.
Controlling Viral Replication
The key safety feature centers on the virus’s reliance on a specific molecule—a protease—provided exclusively by the bacteria. Since the bacteria naturally reside only within the tumor microenvironment, this protease is unavailable elsewhere in the body. As a result, even if the virus were to escape the confines of the tumor, it would lack the necessary component to continue replicating in healthy tissue.
This synthetic dependence creates a two-layer safety net: first by ensuring targeted delivery, and second by preventing accidental viral spread outside the tumor. The result is a system that significantly reduces the risk of harming healthy cells, making the therapy a promising candidate for clinical use.
Table: Safety Features of the Bacterial-Viral System
| Safety Feature | Description |
|---|---|
| Protease-Dependent Viral Maturation | Virus only matures in the tumor environment where the bacteria are present, preventing uncontrolled replication. |
| Localized Bacterial Activity | Bacteria naturally reside in the tumor due to low oxygen and nutrient-rich conditions. |
| Self-Destruction of Bacteria | Bacteria lyse within the tumor to release the virus, ensuring targeted viral activation. |
By embedding these controls into the therapy, the scientists have created a method that not only targets cancer more effectively but also manages the nerve-wracking risks often associated with processing live viruses in the human body.
Bridging Synthetic Virology and Bacterial Engineering: The Future of Multi-Organism Therapies
This research represents a striking milestone in the fusion of synthetic virology with bacterial engineering. In essence, it is the first known example of directly engineered cooperation between two distinct types of microorganisms for therapeutic purposes. What makes this development particularly compelling is its potential to combine the best traits of both worlds.
Advantages of a Multi-Organism Approach
Historically, cancer therapies have struggled with either efficient targeting or safe delivery mechanisms. The bacterial-viral strategy addresses these issues by:
- Enhancing Target Specificity: Bacteria naturally find the tumor, and viruses efficiently infect cancer cells, making the approach a potent duo.
- Balancing Efficacy and Safety: Safety measures, such as protease-dependence and localized activation, ensure that the harmful effects are restricted to the tumor.
- Adaptive Flexibility: Researchers are optimistic about extending this method to different types of cancers, various mouse models, and even different viruses and bacterial strains that have already demonstrated safety in initial trials.
By getting into the nitty-gritty of this technique, experts believe that such multi-organism therapies could eventually become a personalized treatment platform. They offer the possibility of “tuning” the therapeutic agents to better suit the unique characteristics of different tumor types—a prospect that is both exciting and immensely promising.
Looking Ahead: Clinical Applications and Future Research
While the current findings, validated in mice, are promising, the path toward clinical translation is filled with both opportunities and tricky parts. The research team is already working on moving this technology out of the lab and into clinical trials. The next steps involve:
- Testing Across Multiple Cancer Types: Researchers are exploring how this system performs against different tumors, from common solid tumors to more resistant forms of cancer.
- Optimizing Viral and Bacterial Strains: Future work includes investigating various strains that might offer even better targeting or safety profiles.
- Developing a Versatile Toolkit: The goal is to create a range of viral therapies that can adapt to and sense the subtle details of intracellular environments.
The team’s ambition is clear: they aim to develop a comprehensive treatment toolkit that responds to specific conditions inside cancer cells. This adaptive approach could revolutionize how we think about, and treat, cancer, providing a much-needed alternative to conventional therapies that too often come loaded with side effects and limited efficacy.
Expert Perspectives and Broader Implications for Cancer Therapy
The development of this bacterial-viral system has generated excitement in the research community, offering a clever solution to some enduring issues in cancer therapy. Experts in both synthetic virology and biomedical engineering are beginning to weigh in on its potential impact.
Diverse Opinions Among Specialists
While the majority praise the innovation for its creative solutions, some specialists remain cautious. They note that even though the system has shown promise in animal models, translating these findings into human patients will require further rigorous studies. Key concerns include:
- Scalability: Whether the approach can be reliably reproduced in larger-scale human trials, given the nerve-wracking variability between species.
- Long-Term Effects: Understanding any potential side effects or complications that could result from manipulating live microorganisms to treat cancer.
- Regulatory Hurdles: Developing comprehensive guidelines that ensure patient safety while embracing innovative therapeutic techniques.
These voices underscore the importance of both optimism and pragmatism in evaluating new treatments. While the current system represents one of the most technically advanced and novel platforms to date, health professionals stress that further research is critical for ensuring its safety and efficacy in humans.
The Broader Implications for Medical Science
The potential impact of this research extends far beyond just one new therapy. If successfully translated into clinical practice, it could represent a significant turning point in our approach to combating cancer. Here are a few ways this innovation might shape the future of medicine:
- Expanding the Therapeutic Toolbox: Combining different microorganisms introduces the idea of multi-organism therapies, which could be applied to various diseases beyond cancer.
- Personalized Medicine: The ability to tailor therapies based on the precise characteristics of a tumor’s microenvironment paves the way for personalized treatment strategies.
- Improved Safety Profiles: Built-in safety measures increase the chances that treatments will target only the diseased cells, reducing the risk of damage to healthy tissue.
Overall, this cooperative strategy stands as an important example of how creative engineering and a willingness to work through the tangled issues of microbial behavior can lead to innovative solutions in modern medicine.
Addressing Concerns and Future Challenges in the Field
Despite the promising results and potential advantages, it is important to acknowledge the challenges that lie ahead. In any rapidly evolving field like oncolytic viral therapy, there are several confusing bits that need further clarification and additional research before the technology can be broadly applied in clinical settings.
Technical and Biological Hurdles
Several technical challenges remain, including understanding the delicate interplay between bacteria and viruses at the molecular level. Researchers must get around the complicated pieces of ensuring that the bacterial carriers do not invoke unintended host responses, and that the virus remains under strict control throughout its therapeutic cycle.
Some of the key challenges include:
- Optimizing Dosage: Finding the right balance between bacterial load and viral concentration is super important for efficacy and safety.
- Managing Host Responses: While the bacteria hide the virus from antibodies, the immune system may still react to the bacterial carriers in unexpected ways.
- Preventing Unintended Spread: Even with the engineered safeguards, consistent monitoring is needed to ensure that the virus does not inadvertently migrate beyond its intended habitat.
Each of these areas represents a field on edge with opportunities for improvements, requiring close collaboration between engineers, biologists, and clinical experts to figure a path toward safer application.
Addressing Regulatory and Ethical Considerations
Novel therapies that combine multiple biological agents also raise ethical and regulatory questions. Regulatory agencies need to work through the fine details of evaluating these living medicines, which differ significantly from traditional drugs. The areas that need particular attention include:
- Clinical Trial Design: Ensuring robust trial protocols that can assess long-term outcomes and manage any side effects.
- Risk Assessment: Continuous evaluation of the risk of bacterial or viral escape and its potential impact on the patient’s overall health.
- Ethical Approvals: Addressing any questions regarding the use of genetically modified organisms in medicine, particularly related to environmental exposure and biosafety.
Both regulatory bodies and research institutions are already working through these nerve-racking issues to find a balanced framework that supports innovation while maintaining patient safety as the utmost priority.
The Role of Synthetic Virology in Modern Cancer Research
This approach is a prime example of how synthetic virology is reshaping cancer treatment—merging principles of genetic engineering with advanced biological systems. By diving in to explore the fine shades of virus engineering, scientists have been able to design a system that is both precise in targeting and robust in its therapeutic action.
Advancements in Viral Engineering
The oncolytic virus used in these studies isn’t a random choice; it has been specifically engineered to work in tandem with its bacterial partner. Some of its key features include:
- Enhanced Tumor Selectivity: The virus is programmed to replicate primarily within cancer cells, reducing off-target effects.
- Self-Limiting Replication: Thanks to the dependency on bacterial protease for maturation, the virus is prevented from spreading in non-target tissues.
- Adaptive Activation: The viral genome has been fine-tuned to sense the unique conditions of the tumor microenvironment before activation.
These advancements underscore how synthetic virology is moving beyond conventional paradigms, striving to resolve the tricky parts of cancer treatment with creative molecular designs that get into the small distinctions between healthy and malignant tissues.
Integrating Synthetic Biology with Clinical Needs
It is one thing to create a promising laboratory model; it is another to translate that model into a therapy that can be used safely and effectively in patients. The research team’s success in mice represents only the first step in what will likely be a long process of clinical testing and refinement.
By combining synthetic biology with the clinical demand for effective and safe cancer therapies, this approach opens up possibilities for:
- Next-Generation Therapies: Future treatments could seamlessly integrate other therapeutic payloads or even immune modulators alongside oncolytic viruses.
- Personalized Protocols: Adjustments in bacterial or viral strains may eventually allow clinicians to customize treatments based on an individual patient’s tumor profile.
- Enhanced Combination Therapies: The system could be combined with existing chemotherapy or immunotherapy regimens to create a multifaceted attack against cancer.
While there are many little twists to unravel regarding precise clinical implementation, the fusion of synthetic virology and bacterial engineering stands as a testament to the innovative future of cancer treatment.
Patient-Centered Perspectives: What This Means for Those Affected by Cancer
For patients battling cancer, the development of novel therapies is a beacon of hope. Although the journey from the laboratory to the clinic is long and on edge, many patient advocates see the potential benefits of this dual-action system as revolutionary.
Addressing Patient Safety and Quality of Life
One of the most appreciated aspects of this new therapy is its focus on safety. By delivering the therapeutic virus directly into the tumor and limiting its spread, there is potential to reduce the side effects commonly associated with conventional cancer treatments such as chemotherapy and radiation.
Some of the patient-centered benefits include:
- Reduced Systemic Toxicity: The virus is shielded and activated only within the tumor, potentially sparing healthy tissue from harmful side effects.
- Improved Targeting: The combination of bacterial homing and viral replication ensures that the treatment zeroes in on cancer cells specifically.
- Potential for Combination Approaches: This therapy might be integrated with other treatments to create a comprehensive plan tailored to each patient’s unique situation.
For many, a treatment that can offer targeted destruction of cancer cells while preserving overall quality of life is a significant advance. Such innovations provide hope against a backdrop of treatments that are often as intimidating as they are necessary.
Engaging Patients in the Translation Process
It is also crucial for patients and caregivers to be engaged in the conversation about emerging therapies. Transparency regarding treatment risks, trial phases, and anticipated outcomes builds trust and helps manage expectations. As clinicians and researchers work through the tangled issues of experimental design and safety protocols, patients deserve clear and accessible information about these novel treatments.
Patient advocacy groups play a key role in this dialogue, ensuring that the needs and concerns of those affected by cancer are heard. By staying informed and asking critical questions, patients can help guide future research priorities, making sure that advancements in therapy translate into real-world benefits.
Future Directions and the Path to Clinical Adoption
The promising results seen in animal models have paved the way for further exploration. However, before this bacteria-virus therapy becomes a standard treatment option, several nerve-racking steps must be undertaken.
Designing Rigorous Clinical Trials
Transitioning from a controlled laboratory setting to complex human biology involves carefully constructed clinical trials that account for the small distinctions between patients. Key aspects of these trials will include:
- Phase I Safety Trials: Assessing the immediate safety and tolerability of the therapy in humans.
- Phase II Efficacy Trials: Evaluating how well the treatment works across different cancer types and patient populations.
- Phase III Comparative Studies: Comparing the new treatment with existing standards to determine overall benefits and risks.
Each phase will require scientists and clinicians to work closely together, troubleshooting any confusing bits that arise while optimizing dosing, delivery, and efficacy. Overcoming these challenges is key to ensuring that the therapy can be safely and effectively administered in a clinical setting.
Collaborative Research and Interdisciplinary Efforts
The success of such a multifaceted approach relies on the cooperative efforts of various experts—from engineers and molecular biologists to clinicians and regulatory specialists. Collaboration is essential for:
- Refining the Technology: Continuous improvements to both the bacterial carrier and viral payload will be needed to maximize efficacy and minimize risks.
- Addressing Safety Concerns: Working through each of the complicated pieces with close interdisciplinary communication ensures that the therapy meets the highest safety standards.
- Streamlining Clinical Adoption: By involving regulatory bodies early in the process, researchers can help smooth the path toward approval and clinical use.
Interdisciplinary collaboration ultimately makes it possible to figure a path through the challenging maze of clinical translation, ensuring that each step is taken with patient safety as the ultimate goal.
Concluding Thoughts: A Promising Future for Cancer Treatment
This innovative bacterial-viral therapy represents an exciting frontier in cancer treatment. By capitalizing on the natural tumor-seeking properties of bacteria and the potent cancer-killing abilities of oncolytic viruses, researchers have designed a system that deftly navigates around the immune system’s nerve-wracking barriers while maximizing targeted treatment efficacy.
While it is important to recognize the complicated pieces that still need to be addressed, the early results and the underlying science offer a promising look at what the future may hold for cancer patients. The dual-action strategy, with its built-in safety features and adaptive delivery mechanisms, stands as a testament to the power of innovation in addressing some of modern medicine’s trickiest parts.
It is an exciting time for oncologists, biologists, and patients alike as this research continues to evolve. The potential to refine and eventually adopt this therapy in clinical settings could revolutionize how we treat cancer, opening new avenues for personalized medicine that specifically target the disease while minimizing unwanted side effects.
Key Takeaways for the Medical Community and Patients
- Innovative Combination: The use of bacteria as Trojan horses to deliver oncolytic viruses offers a fresh take on targeted cancer therapy.
- Immune Evasion: The bacteria effectively shield the virus from the body’s natural antibodies, ensuring that the therapy can reach the tumor.
- Localized Treatment: Safety mechanisms built into the viral design prevent it from replicating outside the tumor, significantly reducing potential risks.
- Future Prospects: Extensive clinical trials and interdisciplinary research are essential next steps, promising a future where such therapies could dramatically reshape cancer treatment.
Overall, while there are still several nerve-racking challenges to overcome before this therapy can become a standard clinical tool, the innovative approach of combining bacterial engineering with synthetic virology marks a transformative step forward. As research continues to dip into the subtle details of microbial cooperation, we may soon witness the dawn of a new, more effective era in cancer treatment—one that offers hope, precision, and a better quality of life for patients battling this formidable disease.
In conclusion, as the scientific community figures a path through the twists and turns of integrating advanced biotechnology with clinical applications, this study represents both a milestone and a starting point. By embracing the collaborative power of different microorganisms, we are now witnessing what might be the future of targeted, safe, and effective cancer therapy—a future where even the most daunting challenges can be overcome with courage, ingenuity, and a touch of nature’s own brilliance.
Originally Post From https://www.sciencedaily.com/releases/2025/08/250816113522.htm
Read more about this topic at
Startup’s Trojan Horse Therapy Attacks Aggressive …
The “Trojan Horse” Approach to Tumor Immunotherapy