FDA’s Bold Move to Report Adverse Events Daily
The recent announcement by the Food and Drug Administration (FDA) that it will now publish adverse event data on a daily basis is a significant shift from the former quarterly reporting schedule. This change is not just a routine update; it signals an important transformation in how safety information is shared with the public. In this editorial, we’ll get into the details of this move, look at the potential benefits for public health, and discuss some of the tricky parts and tangled issues that might arise as a result of this new system.
Enhancing Public Health Through Daily Reporting
Traditionally, the FDA relied on quarterly reporting through its Adverse Event Reporting System (FAERS). While periodic updates provided a structured approach, they often left a gap between data collection and public dissemination. By moving to a real-time, daily reporting system, the FDA aims to reduce the delay in identifying safety signals for drugs and biologics, thereby protecting public health more promptly.
Boosting Transparency in Drug Safety Monitoring
Transparency in healthcare is more than just a buzzword—it’s a cornerstone of trust between the public, healthcare professionals, and regulatory agencies. The daily updates allow stakeholders to get unprecedented access to information about adverse events and medication errors. These updates encourage medical professionals and consumers alike to question, analyze, and learn from the data as soon as it is available.
This clearer picture of the safety profile of drugs and biologics can lead to:
- Improved monitoring of drug reactions
- Swift action to remove problematic medications from the market
- Enhanced discussions between doctors and patients about treatment options
Confronting the Tricky Parts: Data Overload and Misinterpretation
While the move toward more frequent data updates is largely welcomed, it comes with challenging bits that cannot be ignored. With daily data publication, there’s a genuine risk of health professionals and the general public misinterpreting numbers or drawing erroneous conclusions from raw data.
Consider these points:
- The sheer volume of data may be overwhelming for those not accustomed to daily feeds.
- The absence of contextual analysis can make it tough for non-experts to differentiate between significant safety signals and routine adverse events.
- Rapid data release might occasionally result in the public getting anxious about minor issues that, in a broader perspective, are well within acceptable limits.
To help steer through these challenges, it is essential that the FDA and allied experts provide clear guidance and interpretation alongside the raw data.
Modernizing the Safety Monitoring Infrastructure
The FDA’s decision is part of a broader initiative to modernize its data collection and monitoring infrastructure. By increasing the frequency of adverse event reporting, the agency is aiming to address some of the subtle parts that make the current system less responsive to emerging safety issues.
Streamlining the Adverse Event Reporting Process
FDA Commissioner Martin Makary, MD, MPH, emphasized that adverse event reporting should be fast, seamless, and transparent. For too long, stakeholders navigating clunky, outdated reporting mechanisms have had to deal with a nerve-racking wait for months before safety data became public. The revamped system calls for a smoother process from the moment an event is reported to when it reaches the public domain.
This transformation involves several key adjustments:
- Integration of new IT solutions to handle large quantities of data.
- Restructuring of data analysis techniques to filter out noise and highlight important safety signals.
- Enhanced communication channels that allow for the quick dissemination of critical information.
Capitalizing on Advanced Technology
The move towards daily reporting aligns with larger trends in the medical industry that rely on advanced data analytics and machine learning. These technologies can help sift through large datasets to identify patterns that might be lost in a static, quarterly report. In doing so, the agency can more effectively manage the overwhelming amounts of data and glean the subtle details that matter in real-world scenarios.
Some of the benefits of incorporating advanced technology include:
- Real-time analysis that helps expedite decision-making processes.
- Automated systems to flag urgent safety signals, thus aiding healthcare providers in taking timely preventive measures.
- Improved accuracy in data collection, reducing the risk of erroneous events going unnoticed.
Implications for Healthcare Professionals and Patients
The shift to daily adverse event reporting is not just a bureaucratic change—its implications extend to the day-to-day operations of healthcare providers and the safety of patients. As more detailed and timely data becomes available, both groups stand to benefit.
Empowering Healthcare Providers
For healthcare providers, having access to up-to-date safety data is key to making informed decisions about patient care. Clinicians can now find their way around a more dynamic information landscape, which helps them:
- Quickly spot unusual patterns and trends in adverse drug reactions.
- Adjust their treatment plans based on fresh insights.
- Engage in more informed discussions with patients about medication risks and benefits.
This democratization of data is super important for building trust and ensuring that medical decisions are made on the best available evidence. However, there is also a responsibility on the side of healthcare professionals to interpret this data with caution, avoiding knee-jerk reactions to every new data point.
Guidance for Patients and Consumers
Patients and consumers, who are now just a few clicks away from detailed adverse event data, might find themselves facing intimidating and sometimes confusing bits of information. While the move towards daily reporting is intended to facilitate greater transparency, it also requires the public to take a more active role in understanding drug safety.
Some steps that can help consumers make sense of the new system include:
- Seeking guidance from trusted healthcare professionals who can explain the context behind the figures.
- Using reputable online resources to understand what counts as a significant adverse event versus a minor side effect.
- Participating in community discussions to share experiences and insights regarding medication safety.
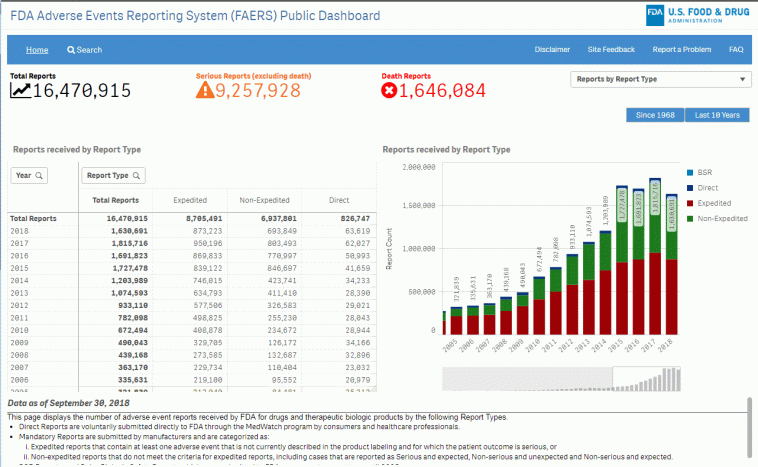
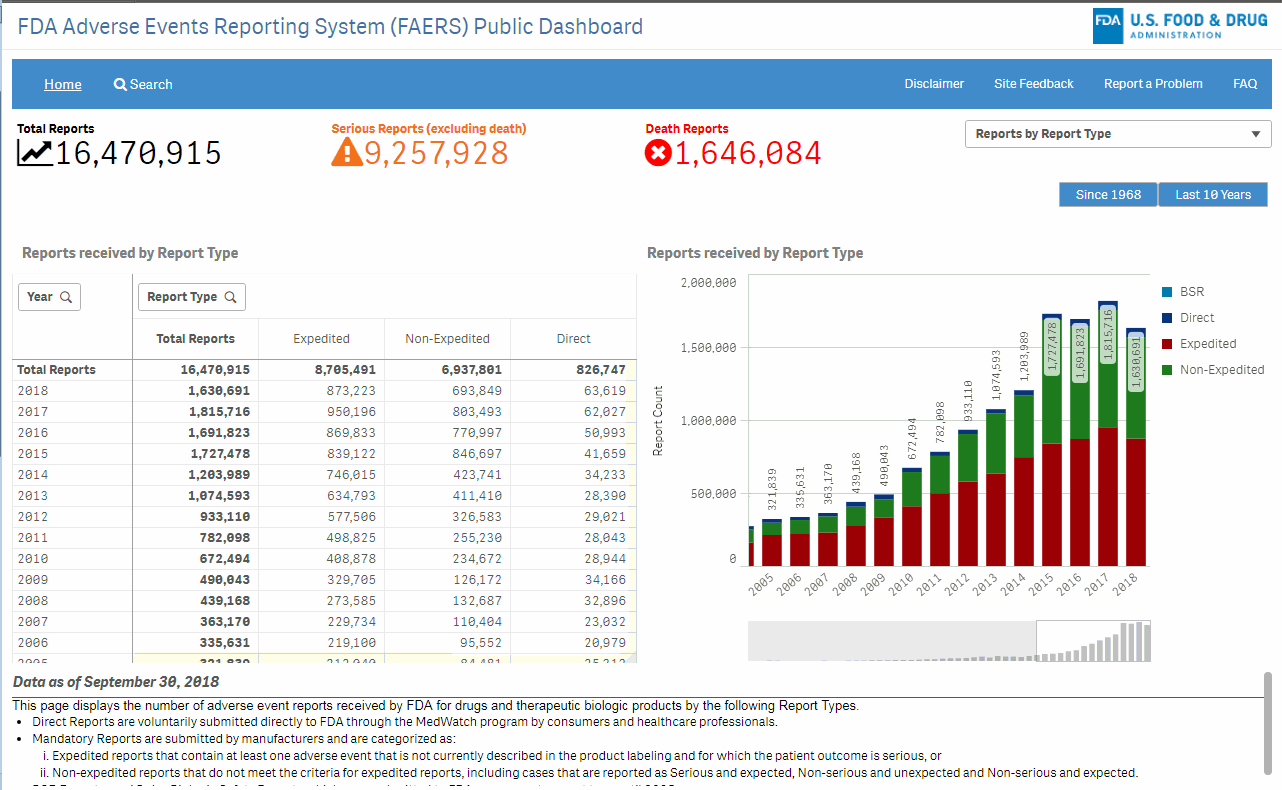
The Role of the FDA’s Primary Reporting Database (FAERS)
The FDA Adverse Event Reporting System (FAERS) is the backbone of this new initiative. As the agency’s primary database, FAERS collects and analyzes adverse event data stemming from various sources, including healthcare professionals, consumers, and drug manufacturers. Its role is more critical now than ever.
What FAERS Brings to the Table
At its core, FAERS is designed to capture vast amounts of data related to drug safety. With the new daily reporting mandate, the system is expected to:
- Accelerate the identification of potential safety signals.
- Expose hidden complexities in the adverse event data that may be overlooked in quarterly reports.
- Facilitate proactive regulatory action when unexpected trends are detected.
It is essential, though, that stakeholders understand the limitations of FAERS data. The reported figures represent cases where an adverse event has been noticed, but that does not necessarily imply causation. In other words, while the data may show a correlation between a drug and an event, it is not always a clear-cut case of cause and effect.
Interpreting Daily Data: A Shared Responsibility
Interpreting data from FAERS is a shared responsibility among the FDA, healthcare professionals, and consumers. To ensure that the provided information is used constructively, all parties must work together to:
- Understand that raw data needs to be analyzed within context.
- Recognize that some adverse events may be coincidental rather than directly drug-induced.
- Avoid overreaction to isolated data points without considering broader trends.
By fostering an environment of critical discussion and careful interpretation, the new daily reporting system can fulfill its promise of enhanced safety monitoring without causing unnecessary alarm.
Opportunities for Improved Patient Safety and Modern Medical Practices
The shift from quarterly to daily data publication presents a unique opportunity to improve patient safety and modernize medicine. The benefits of this evolution extend far beyond mere transparency—this is about equipping both healthcare professionals and patients with timely, actionable insights.
Strengthening Drug Surveillance Protocols
The healthcare system will likely see a significant enhancement in its capability to surveil drugs more effectively. Here are a few reasons why this change will be beneficial:
- Early Signal Detection: The daily reports can quickly highlight potential safety issues that might otherwise be delayed in a quarterly cycle.
- Real-Time Updates: Constant updates mean that once a new safety concern is spotted, investigations and interventions can be launched more swiftly.
- Continuous Improvement: With ongoing surveillance, the pharmaceutical industry is incentivized to maintain and improve the safety profiles of its products.
This proactive approach can potentially lead to more rapid removal or correction of problematic medications, reducing the risk of harm to patients.
Modern Medicine in a Digital Age
The advent of daily reporting aligns seamlessly with the digital transformation that is shaping modern medicine. In an era where data drives decision-making, modernizing safety monitoring practices is not just about reporting numbers—it is about creating a culture of quick response and continuous improvement.
Some of the broader impacts of this digital shift include:
- Increased trust in regulatory processes, as data becomes more accessible and timely.
- Better collaboration among international health agencies, as real-time data helps synchronize global safety responses.
- Empowerment for patients to be more involved in their healthcare decisions by providing them with the latest information.
Navigating the Confusing Bits in Data Interpretation
It is important to acknowledge that while the daily reporting system is a leap forward, it comes loaded with issues that require careful handling. The transition period, in particular, might be a nerve-racking time for both medical professionals and the public as they adjust to this new influx of information.
Potential Pitfalls of Rapid Data Dissemination
Some of the potential challenges include:
- Data Misinterpretation: Without proper context, it may be difficult to distinguish between routine adverse events and significant safety signals.
- Information Overload: The daily flow of data might overwhelm some stakeholders, making it harder to find the fine points that truly matter.
- Public Anxiety: Rapid updates might inadvertently lead to heightened public fear if isolated events are blown out of proportion.
To manage these twists and turns effectively, a concerted effort from the FDA and healthcare communities is needed. It is super important that they offer educational resources and contextual analysis so that everyone can figure a path through the daily data updates without undue stress.
Strategies for Effective Communication and Education
The success of this initiative will depend largely on how effectively the information is communicated. Here are some strategies that can help:
| Strategy | Benefit |
|---|---|
| Regular Webinars | Provide healthcare providers with guidance on interpreting daily data. |
| Clear Visualizations | Infographics and charts can make data easier to understand for non-experts. |
| User-Friendly Dashboards | Interactive platforms can allow users to explore data with explanation notes on crucial points. |
| Official FAQs | Detailed question-and-answer sections can help clarify common concerns about the data. |
By implementing these strategies, the FDA can help both professionals and the public navigate the overwhelming volume of daily information with more confidence and clarity.
How Daily Reporting Fits into the Broader Healthcare Landscape
The move towards daily reporting is not an isolated event but part of a broader trend in healthcare to embrace digital technologies and data-driven decision-making. This shift has several implications for modern medicine and patient-centric care.
Enhancing Integrated Healthcare Systems
Modern healthcare increasingly relies on integrated systems, where data flows seamlessly between various stakeholders—from research laboratories to patient care providers. Daily adverse event reporting is a key component of this integrated approach because:
- It breaks down silos: More frequent data sharing encourages collaboration across departments and institutions.
- It facilitates rapid risk assessment: Immediate access to updated data can help healthcare systems quickly pinpoint and address safety concerns.
- It supports evidence-based practices: Clinicians have access to the most current data, reinforcing the need for practices that reflect the latest insights.
These improvements not only bolster patient safety but also foster an environment where innovation and quality care go hand in hand.
Global Perspectives on Drug Safety Reporting
While the FDA’s move is a domestic policy development, it has global implications. With many international health agencies closely monitoring drug safety data, the enhanced transparency and speed of the U.S. system could serve as a model for other nations. Key points include:
- Improved international collaboration through shared data and joint safety initiatives.
- Potential for harmonized global safety standards that protect consumers worldwide.
- An opportunity for large-scale data studies and cross-border research to identify trends in adverse events.
This global perspective underscores the necessity for all health authorities to consider modernizing their data reporting practices to keep pace with technological advances and the rising expectations of both healthcare professionals and the public.
The Road Ahead: Balancing Transparency with Responsible Reporting
As we take a closer look at the landscape of drug safety and adverse event reporting, it becomes clear that the journey is only just beginning. The commitment to daily reporting is a promising development, yet it must be balanced with responsible data interpretation and communication strategies.
Responsible Use of Daily Data
The daily reporting system should be seen as a tool for empowerment rather than a source of constant worry. To ensure that everyone gets around the information effectively, the following principles should guide the use of daily data:
- Context is key: Data should always be presented with background context, allowing for a more nuanced understanding of the events reported.
- Collaboration: Regulators, healthcare providers, and researchers must work together to interpret the data correctly, avoiding knee-jerk reactions to isolated figures.
- Education: Ongoing educational efforts are needed to help the public and professionals figure a path through the intricacies of the data.
Emphasizing responsible use means recognizing that while the daily reports are a super important step toward modernizing drug safety monitoring, they are just one piece of the puzzle in creating a robust, transparent, and effective healthcare ecosystem.
Ensuring the Long-Term Success of Daily Reporting
For daily reporting to truly succeed, several long-term strategies must be put in place:
- Regular Reviews and Audits: Ongoing evaluations of the system’s performance can help identify any areas where additional supportive measures are needed.
- Feedback Mechanisms: Establishing channels through which both healthcare providers and consumers can offer feedback will be essential for continuous improvement.
- Integration with Broader Health Initiatives: Linking the adverse event data with other public health measures can amplify its impact on improving patient safety and healthcare outcomes.
The thoughtful implementation of these strategies will be key to ensuring that daily reporting not only meets its immediate goals but also evolves into a lasting asset for public health policy and regulation.
Concluding Thoughts: A Step Toward a More Resilient Healthcare Future
The FDA’s decision to transition to daily adverse event reporting is a bold and welcome stride towards a more modern, efficient, and transparent healthcare system. By providing near real-time access to critical safety data, this initiative lays the groundwork for faster interventions, better-informed patient care decisions, and a deeper understanding of medication safety profiles.
Nevertheless, as we celebrate this move towards modernization, it is crucial to acknowledge the nerve-racking and sometimes overwhelming nature of handling such vast amounts of data on a daily basis. The potential for data overload is real, and without clear interpretation and context, the fine points of the information could be misread, leading to unnecessary public concern.
Healthcare professionals, regulatory bodies, and even patients have a shared responsibility in ensuring that this wealth of information is not only accessible but also understandable and actionable. Alongside improvements in IT infrastructure and data analytics, there must be parallel efforts in educational outreach and user support. Whether through webinars, user-friendly dashboards, or clear explanatory content, every step taken towards clarifying the ‘tricky parts’ of this data will contribute to its successful long-term adoption.
The move also highlights a broader trend in modern medicine: the growing need to integrate digital advancements with traditional healthcare practices. Real-time data is a game changer in many respects, opening up opportunities for both proactive healthcare management and a more engaged patient community.
Ultimately, this transition to daily reporting may well represent one of the key turning points in the ongoing evolution toward more resilient and responsive healthcare systems. With better data always comes the challenge of interpreting that data within a multifaceted, ever-changing landscape. Yet, with dedicated support, continuous improvement, and a shared commitment from all parties involved, the future of healthcare safety monitoring looks promising.
Key Takeaways
In summary, the FDA’s move to daily adverse event reporting is a significant step with multiple dimensions worth considering:
- Enhanced Transparency: Daily updates increase transparency, ensuring that both the public and healthcare professionals have access to up-to-date drug safety information.
- Improved Monitoring: The rapid data dissemination supports early detection of potential issues, allowing faster, more informed interventions.
- Technological Integration: The new system leverages modern data analytics and IT solutions, setting a precedent for digital transformation in healthcare.
- Challenges to Address: With the benefits come tricky parts such as potential data overload, misinterpretation, and the need for robust public education.
- An Evolving Ecosystem: This initiative reflects a broader trend in integrating digital technologies with healthcare practices, aiming for enhanced patient safety and better-informed clinical decisions.
As we step into this new era of daily reporting, it’s essential for all stakeholders—from regulators and clinicians to concerned consumers—to join forces. By taking the necessary steps to offer clear context, support, and guidance, we can ensure that the benefits of this innovative system are fully realized while minimizing any unintended negative effects.
Final Reflections
In conclusion, while the twists and turns of modernizing drug safety reporting are not without their nerve-racking moments, the FDA’s initiative is a much-needed development in the healthcare sector. With the potential to significantly enhance patient safety through prompt identification of adverse events, this move paves the way for a more responsive, data-driven approach to public health management.
This editorial encourages ongoing dialogue, critical thinking, and cooperative action among all parties involved. By acknowledging the challenges and embracing the benefits, the community can ensure that daily adverse event reporting is not just a momentary trend but a lasting improvement in healthcare transparency and safety.
As we look to the future, may this initiative serve as a model for similar reforms across the globe, leading to safer medications, better therapeutic outcomes, and a healthcare system that is modern, responsive, and truly patient-centered.
Originally Post From https://www.healio.com/news/primary-care/20250822/fda-begins-daily-reporting-of-adverse-event-data
Read more about this topic at
A Daily HSE Observation Report Sample
250+ Free Safety Talks and Toolbox Talk Meeting Topics