Advanced Urothelial Carcinoma Treatment Advances
The recent developments in oncological research have generated a buzz among healthcare professionals and patients alike. A new trial, known as the JAVELIN Bladder Medley, reported promising outcomes with a first‐line switch maintenance strategy using a combination of avelumab plus sacituzumab govitecan in advanced urothelial cancer. In this opinion editorial, we get into the details of the study and share our thoughts on how such combination therapies might change the landscape of cancer care.
This article aims to offer an in‐depth look at the trial results, while weighing the benefits and challenges that come with new treatment modalities. For many, the idea of combining an immune checkpoint inhibitor with an antibody-drug conjugate may seem intimidating. However, if we take a closer look and figure a path through the data, the benefits might outweigh the difficult bits that are inherent to complex treatment decisions.
Combination Therapies: A Promising Strategy in Oncology
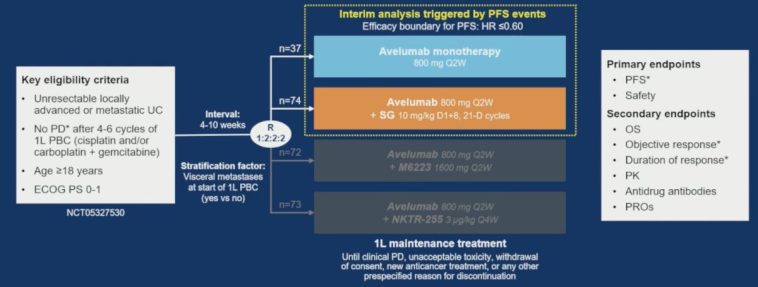
The JAVELIN Bladder Medley trial enrolled patients with locally advanced or metastatic urothelial carcinoma who had not experienced disease progression following platinum-based chemotherapy. The study randomized patients to receive either avelumab alone or in tandem with sacituzumab govitecan. With a median progression-free survival (PFS) improvement of more than 11 months in the combination arm versus a little under 4 months for monotherapy, the study offers compelling evidence that combining immune checkpoint inhibitors and antibody-drug conjugates may be key in boosting patient outcomes.
Understanding the Trial’s Rationale and Design
The researchers aimed to address some of the tricky parts that often complicate effective treatment of urothelial cancer. The rationale behind combining avelumab, a well-known PD-L1 inhibitor, with sacituzumab govitecan, an anti-Trop2 antibody-drug conjugate, was to leverage the strengths of both agents. While avelumab targets immune checkpoints to help the body fight cancer, sacituzumab govitecan aims directly at the cancer cells by delivering cytotoxic agents.
The trial was intentionally designed as an international, open-label, parallel-arm, phase 2 study. With patients enrolled after showing a positive response to frontline chemotherapy, the study ensured that subjects were at least 18 years of age and had an ECOG performance status of 0 or 1. This careful selection aimed to minimize some of the confusing bits that often arise from including highly heterogeneous populations. The trial featured an innovative design element: randomization using a 2:1 ratio, allowing researchers to compare the combination therapy’s safety and efficacy against an established monotherapy treatment.
By incorporating a control arm and additional data from the JAVELIN Bladder 100 trial through propensity score weighting, the study added another layer of credibility when comparing outcomes. This additional step helped figure out small distinctions in patient characteristics that might have influenced the results, such as differences in baseline demographics or responses to initial chemotherapy.
Key Findings and Clinical Insights
The trial’s primary endpoint was investigator-assessed progression-free survival (PFS), while secondary endpoints included overall survival (OS), objective response rate (ORR), and duration of response (DOR). At the time of the interim analysis, the combination arm showed a significant improvement in PFS. The median PFS was 11.17 months for the combination group versus 3.75 months for those receiving avelumab alone—a reduction in disease progression risk by 51%.
This outcome has major implications when considering treatment options for patients with advanced urothelial carcinoma. While the data reveal that the combination therapy significantly extended the period before the disease worsened, it also shed light on some of the nerve-racking aspects of adding another drug to the mix. For example, the treatment-related adverse effects (TRAEs) were more frequent in the combination arm compared to the monotherapy group.
Assessing the Efficacy Metrics
Let’s break down the efficacy metrics in simpler terms. The hazard ratio for progression or death in the combination arm was 0.49, suggesting that patients in this group were nearly half as likely to experience disease progression compared to those on monotherapy. This finding was consistent across several subgroups, reflecting the robustness of the data regardless of small variations in patient characteristics, such as age or metastatic spread.
Moreover, the objective response rates were notably higher in the combination arm, where approximately one in four patients experienced a measurable reduction in tumor size. The median duration of response for the combination therapy reached nearly 12 months, demonstrating not only an improvement in immediate outcomes but also a prolonged benefit over time.
Table: Comparative Efficacy Outcomes
| Parameter | Avelumab + Sacituzumab Govitecan | Avelumab Monotherapy |
|---|---|---|
| Median PFS (months) | 11.17 | 3.75 |
| Risk Reduction of Progression | 51% reduction (HR: 0.49) | Reference |
| Objective Response Rate | 24.3% | 2.7% |
| Disease Control Rate | 68.9% | 43.2% |
This table helps organize the nitty-gritty details of the study outcomes, making it easier to digest the fine points that are essential for understanding the potential benefits of the combination treatment.
Weighing the Safety and Tolerability Concerns
No treatment comes without its complications. While the efficacy numbers paint an optimistic picture, the combination approach did present some tricky parts regarding safety. Treatment-related side effects were higher in the combination arm, with nearly 97.3% of patients experiencing some form of adverse reaction. Moreover, a significant portion of these side effects were grade 3 or higher, meaning they were severe enough to potentially require dose reductions or treatment delays.
Common Side Effects and Their Management
Some of the most frequently reported adverse effects in the combination therapy group included:
- Fatigue
- Diarrhea
- Asthenia, or lack of energy
- Skin-related reactions such as pruritus and rash
- Hematological issues such as neutropenia and anemia
Particularly notable was the incidence of neutropenia—observed in nearly 48% of patients in the combination arm—which can compromise a patient’s ability to fight infections. For clinicians, these side effects represent complex pieces to manage in supportive care, requiring vigilant monitoring and timely intervention. However, when viewed alongside the significant improvements in PFS and objective response rates, many experts believe the trade-off may be acceptable for patients who have limited treatment options after frontline chemotherapy.
Real-World Relevance: Balancing Efficacy with Quality of Life
While the data are promising from an efficacy standpoint, the overall impact on a patient’s quality of life remains a critical consideration. Side effects like fatigue and gastrointestinal issues (e.g., diarrhea and nausea) can substantially affect day-to-day living. Integrating patient-reported outcomes into future studies could help healthcare providers better steer through the challenging parts of treatment while preserving well-being.
Modern oncology is increasingly focused on not just prolonging life but also maintaining a good quality of life during treatment. In this context, the combination therapy’s risk-benefit profile must be weighed carefully. Patients with advanced urothelial cancer often face a nerve-racking prognosis, so treatments that can both extend survival and preserve quality of life are invaluable. Future studies, ideally with longer follow-up periods, will aim to address these small distinctions and help us figure out how best to integrate this approach into routine practice.
Patient Demographics and Treatment Subgroups
Digging into the study further, the patient population in the trial had several characteristics that are worth noting. The median patient age in the combination group was 70 years, compared to 67 in the monotherapy arm. A higher percentage of patients were male, and most participants had primary tumors located in the bladder. Additionally, a majority of patients across both groups were PD-L1 negative, which is an interesting detail given that PD-L1 status sometimes influences responses to immunotherapy.
Subgroup Analysis: What It Means for Personalized Treatment
One of the promising aspects of the trial was that the PFS benefit with the combination therapy was reported across several clinical subgroups. This consistency suggests that the combination treatment might be effective irrespective of certain baseline differences. For clinicians, having a treatment option that works broadly across various patient demographics offers valuable flexibility, especially when managing cases loaded with problematic and tangled issues.
The study also analyzed outcomes based on the type of chemotherapy regimens used before maintenance therapy—whether patients had received cisplatin plus gemcitabine or carboplatin plus gemcitabine. While there were slight differences in response rates, both groups appeared to benefit from the combination therapy, reinforcing its potential application in real-world settings where treatment histories might vary greatly.
Clinical Implications and Future Directions
When evaluating new treatments, it’s critical to weigh both the promise and the challenges. The JAVELIN Bladder Medley trial highlights the potential benefits of using avelumab combined with sacituzumab govitecan as a maintenance therapy in advanced urothelial carcinoma. With a reduction in the risk of disease progression by 51%, the results could alter treatment paradigms if confirmed by further studies.
Impact on Oncology Practice
From an oncologist’s perspective, the study offers several takeaways:
- The combination therapy may serve as a super important treatment option for patients who have responded to first-line platinum-based chemotherapy yet remain at high risk for disease progression.
- It provides additional evidence supporting the integration of antibody-drug conjugates with immunotherapies, a strategy that is gaining traction in oncology.
- The trial design itself, which combined data from different studies using propensity score weighting, offers a model for future research initiatives aiming to compare treatment modalities in a balanced way.
These points are critical for clinicians who need to make informed decisions when managing their patients’ journeys. By taking a closer look at emerging therapies like this combination treatment, physicians can better steer through complex treatment decisions and tailor therapies to individual patient needs.
Research Considerations and the Road Ahead
While the results are encouraging, it is necessary to note that the data on overall survival (OS) remain immature at this stage. This means that longer follow-up periods are needed to understand the complete picture of benefit and risk. Future studies should also incorporate patient-reported outcomes and quality-of-life assessments to fully capture the implications of treatment-related side effects.
Moreover, as combination therapies become more prevalent in oncology, additional research will be needed to address the fine points of dosing, sequencing of treatments, and management of adverse effects. Collaborative efforts across global oncology communities will be essential to refine these approaches further. The ultimate goal will be to develop treatment regimens that maximize benefits while minimizing the small twists and overwhelming challenges associated with toxicity and other complications.
Integration of Novel Agents in First-Line Maintenance Therapy
The results from the JAVELIN Bladder Medley trial represent a strong case for incorporating novel agents in the first-line maintenance setting for advanced urothelial carcinoma. With avelumab already established as a maintenance therapy in prior trials, the addition of sacituzumab govitecan represents a logical next step. This study adds to a growing body of evidence that combining targeted therapies can yield significant improvements in disease management.
Key Considerations for Implementation
Before such combination therapies become part of routine practice, several key factors should be considered by the oncology community:
- Patient Selection: Not every patient will be a candidate for a combination regimen. It is crucial to identify those who are likely to benefit based on factors such as performance status, prior response to chemotherapy, and specific tumor characteristics.
- Dosing Strategies: Finding the right balance in dosing to optimize efficacy while keeping side effects manageable is a significant challenge. Future studies should focus on establishing optimal dosing guidelines.
- Monitoring and Supportive Care: With a high incidence of severe side effects in the combination arm, robust strategies for monitoring and managing these issues will be essential. This includes establishing protocols for dose modifications and supportive interventions for adverse events such as neutropenia and gastrointestinal toxicity.
Addressing these factors will be super important for the successful integration of new therapeutic approaches in oncology practice. Each step will require close collaboration between researchers, clinicians, and patient advocacy groups to ensure that the benefits of these therapies can be realized while mitigating the nerve-racking risks.
Future Research Directions
Looking forward, several research directions merit attention:
- Longer Follow-Up Studies: As mentioned earlier, longer-term data are essential to confirm the observed PFS benefits and to fully appreciate the impact on overall survival.
- Biomarker Research: Identifying biomarkers that predict which patients are more likely to benefit from this combination therapy will be a high priority. This will enable more personalized treatment strategies that can improve outcomes while reducing unnecessary exposure to toxic therapies.
- Quality-of-Life Assessments: Future trials should integrate comprehensive assessments of patient quality of life. Understanding how the benefits of extended PFS compare with the side effect burden can help refine treatment protocols to better serve patients’ needs.
- Real-World Data Integration: Incorporating data from routine clinical practice can provide insights into how these therapies perform outside the controlled setting of a clinical trial. Real-world evidence can highlight both successes and areas needing improvement.
Reflections on the Impact on Patient Outcomes
This study gives rise to important conversations about the future of cancer treatment. For patients suffering from advanced urothelial carcinoma, any advancement that offers the promise of extended survival is welcome news. However, it is equally important for clinicians to balance these advances with the potential for increased side effects. The decision-making process is filled with complicated pieces and twists and turns that require careful consideration.
Patients and caregivers must weigh the potential for improved outcomes against the likelihood of experiencing severe side effects such as significant fatigue, gastrointestinal issues, and hematologic problems. These side effects can be off-putting, making it crucial for healthcare providers to offer comprehensive counseling and supportive care.
Shared Decision-Making in Oncology
The role of shared decision-making in oncology care cannot be overstated. With the emergence of combination therapies that offer strong efficacy signals but also present challenging side effects, it is imperative that oncologists work closely with patients. Discussions about treatment options need to address:
- The potential benefits in terms of extended progression-free survival and overall tumor response.
- The likelihood and nature of adverse events, including strategies for managing these side effects.
- The patient’s values and preferences, particularly regarding quality of life and treatment aggressiveness.
This collaborative approach helps patients feel more in control of their treatment journey and provides reassurance when facing the intimidating reality of cancer treatment. It underlines the importance of a personalized approach, where each treatment recommendation is tailored to the individual’s specific circumstances and needs.
Concluding Thoughts: The Future of Cancer Treatment
As we take a closer look at the JAVELIN Bladder Medley trial results, it becomes clear that combining avelumab with sacituzumab govitecan is a strategy packed with potential. While the efficacy data are promising—with a significantly extended progression-free survival and higher response rates—the management of treatment-related side effects remains one of the more tangled issues.
For the oncology community, these findings reinforce the notion that combination therapies might be the key to unlocking better outcomes for patients with advanced urothelial carcinoma. By carefully weighing the benefits against the challenging adverse effects, and by investing in further research to refine these treatments, the future of cancer care looks brighter from both a survival and quality of life standpoint.
In sum, while there are still many small details to sort out, this study offers a positive hint of what might be possible when we combine modern immunotherapies with targeted antibody-drug conjugates. As healthcare providers digest these findings and make their way through the evolving treatment landscape, the hope is that these tools will empower them to provide more effective, personalized care for patients facing one of the most intimidating diagnoses in oncology.
Key Takeaways for Clinicians and Patients
To wrap up this discussion, here are some of the critical points to remember:
- Improved Progression-Free Survival: The combination therapy significantly extended PFS, reducing the risk of disease progression by 51% when compared to avelumab alone.
- Higher Response Rates: Patients receiving the combination therapy showed notable improvements in tumor response, which is encouraging for those who had already responded well to frontline chemotherapy.
- Management of Side Effects: While adverse effects were more common and severe in the combination arm, early recognition and management through supportive care protocols are essential.
- Personalized Care: The data underscore the importance of shared decision-making, ensuring that treatments are aligned with patient values and individual clinical circumstances.
- Ongoing Research: Longer-term studies and additional quality-of-life analyses will be critical in refining how these combination therapies are used in routine clinical practice.
In conclusion, by embracing these advances and continuing to take a closer look at the evidence, oncologists and patients alike can hope to witness a turning point in the management of advanced urothelial carcinoma. The journey ahead, although filled with some confusing bits and nerve-racking moments, holds the promise of improved outcomes and enriched lives for those affected by this challenging disease.
The balancing act between efficacy and safety is a constant companion in the field of oncology. With the progress seen in the JAVELIN Bladder Medley trial, the integration of combination therapies offers a new chapter—one where meticulous patient selection, tailored dosing, and proactive management of side effects are more critical than ever. As we continue to navigate the evolving landscape of cancer treatment, these findings serve as both an inspiration and a reminder of the intricate, yet rewarding, nature of modern oncology.
Ultimately, while further studies are needed to cement these findings and broaden our understanding, current evidence indicates that this approach might become a cornerstone in the treatment of advanced urothelial carcinoma. For now, the oncology community must stay informed, remain agile in its treatment strategies, and above all, keep the patient’s quality of life at the forefront of all decisions.
Originally Post From https://www.onclive.com/view/frontline-maintenance-avelumab-plus-sacituzumab-govitecan-extends-pfs-in-urothelial-cancer
Read more about this topic at
Breakthrough Clean Technologies®
Vision Series – Cleaning Kits