Metabolism and Epigenetics in Cancer: Toward Personalized Treatment
The interaction between the body’s metabolic processes and the epigenetic controls over gene expression is a field bustling with promising research insights. In recent years, researchers from across the globe have taken a closer look at the tangled issues governing cancer biology. This discussion aims to figure a path through the maze of how metabolism and epigenetic changes call the shots in cancer’s development and treatment, offering a fresh perspective on personalized care through diet changes and targeted therapies.
Drawing from over 500 previous studies, clinical insights, and hands-on experiments in both human cell lines and animal models, experts are beginning to piece together a picture of how glucose and lipid reprogramming—plus numerous tricky parts of metabolic pathways—affect cancer progression. This editorial will poke around the fine points of these intertwined systems, examine how these complex yet fascinating processes influence disease management, and propose an informed look at individualized treatments for cancer.
Understanding the Connection Between Metabolism and Epigenetic Regulation
At its core, epigenetic change focuses on modifications that regulate gene expression without altering the actual DNA sequence. These changes include DNA methylation, chromatin remodeling, and histone modifications. The fine shades that distinguish healthy cells from cancer cells might appear at first as subtle details; however, once you dig into the research, the small distinctions between metabolic adjustments and epigenetic modifications become strikingly clear.
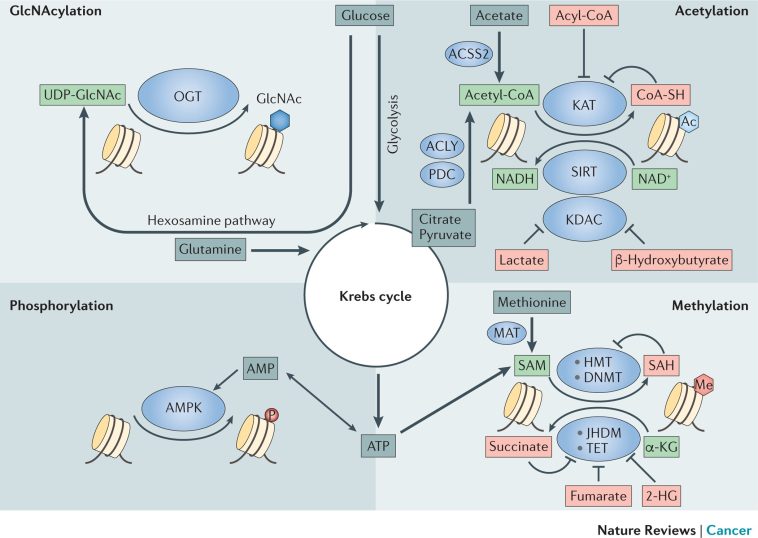
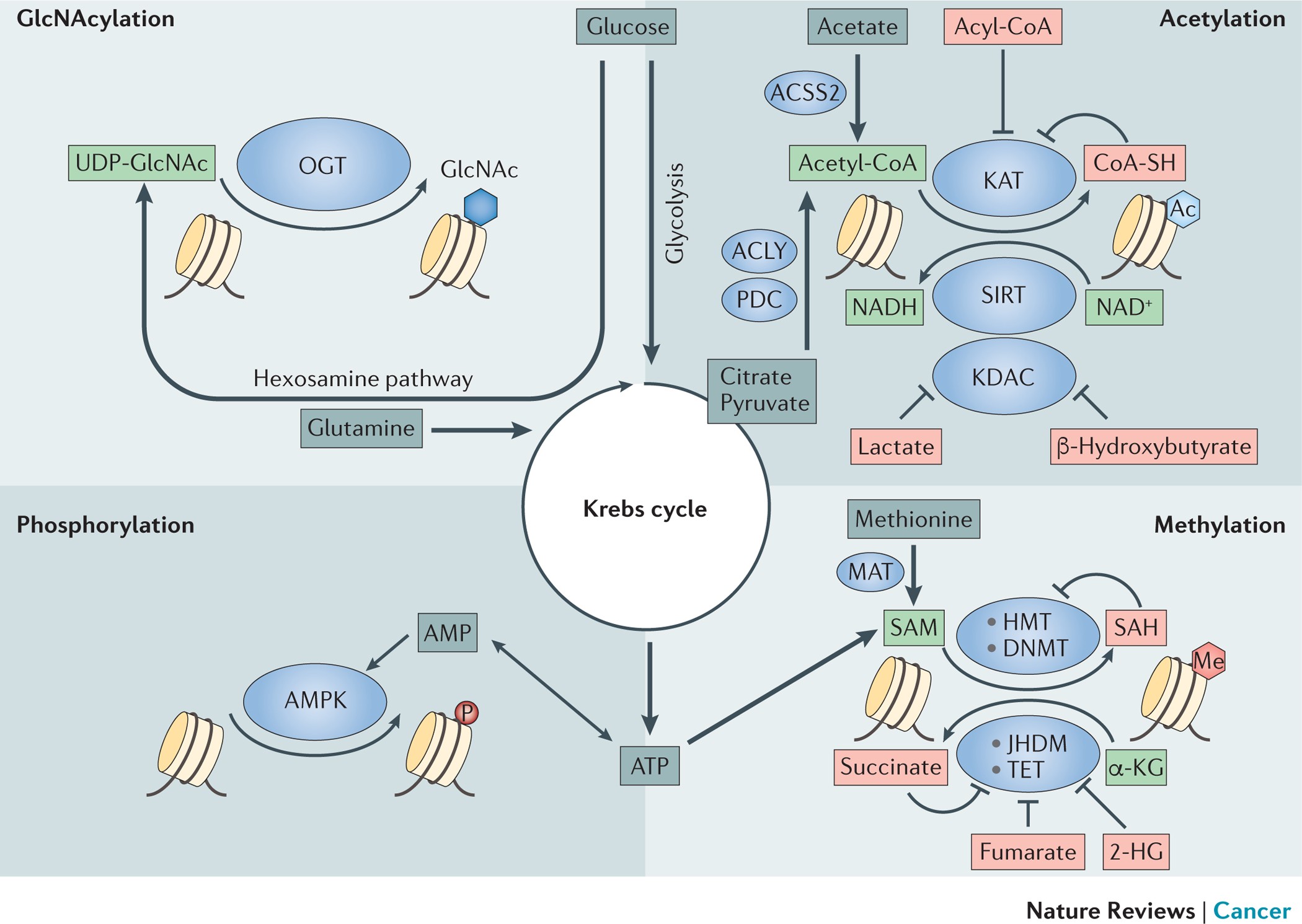
It is becoming ever more apparent that many genes involved in glucose metabolism, lipid processes, energy production, and the modulation of hormones are heavily influenced by these epigenetic switches. In a trend that has evolved over recent years, scientists have started to notice that the specific metabolites created by our body, and even those produced by our gut flora, serve as coenzymes or substrates in the epigenetic modification process.
This duality—that metabolism influences epigenetics and epigenetic changes, in turn, modify metabolic pathways—creates a feedback loop crucial to understanding cancer. In essence, the body’s metabolic activities are not solely responsible for providing energy or constructing vital molecules; they also subtly fine-tune gene expression, supporting tumor growth under certain conditions.
A Closer Look at the Metabolic Reprogramming in Cancer
Metabolic reprogramming is one of the hallmark features of cancer. Tumor cells often shift their energy production methods, favoring rapid glucose consumption and altered lipid metabolism. Such shifts are not random; they represent a strategic adaptation to support uncontrolled cell growth and survival in an ever-changing tumor microenvironment.
This process may initially seem overwhelming and off-putting due to the nerve-racking number of factors at play. However, by breaking it down into smaller, more manageable pieces, we can figure a path through the confusing bits of metabolic alterations in cancer cells.
- Glucose Metabolism: Tumor cells tend to rely more on glycolysis—a process that converts glucose to lactate—even when oxygen is available. This phenomenon, known as the Warburg effect, provides the rapidly growing cells with quick energy and building blocks required for proliferation.
- Lipid Metabolism: Changes in fatty acid synthesis and degradation supply tumor cells with essential membrane components and energy. These shifts ensure that cancer cells have a steady stream of lipids both for signaling and structural purposes.
- Energy Production: Mitochondrial function often differs in cancer cells. The metabolic reprogramming allows these cells to pivot between oxygen-rich and oxygen-poor conditions, stitching together a survival strategy that makes targeting them more complicated.
In clinical settings, understanding the key twists and turns of these metabolic processes has revealed potential targets for intervention. Therapies that focus on blocking specific metabolic pathways might help starve tumor cells of their necessary resources, while the addition of dietary interventions could modulate the availability of essential metabolites.
The Role of the Tumor Microenvironment in Epigenetic Changes
Cancer does not develop in isolation. The tumor microenvironment, which includes surrounding immune cells, blood vessels, and even microbes, plays a critical role in shaping epigenetic modifications. In recent studies, alterations in this microenvironment have shown a strong association with how glycemic and lipid-focused metabolic networks change and, subsequently, how epigenetic drivers are activated.
For instance, metabolites produced by the gut flora and by neighboring cells in the tumor vicinity can either amplify or moderate epigenetic marks. This two-way communication means that changes in a patient’s metabolism and diet might not only impact overall physical health, but may also influence cancer progression on a molecular level.
Some of the confusing bits include:
- Metabolite Influence: Metabolites such as acetyl-CoA or S-adenosylmethionine, which serve as coenzymes for epigenetic modifications, can shift the balance of gene expression.
- Environmental Cues: Hypoxic conditions (low oxygen levels) or acidic microenvironments may trigger subtle parts of epigenetic changes, further altering cancer behavior.
The above reflects the complexity of the relationship between the tumor microenvironment and epigenetic regulation. By taking a closer look at these factors, researchers can better understand not just the biological underpinnings of cancer but also potential avenues for therapeutic intervention.
Experimental Foundations: Cell Lines, Animal Models, and Clinical Studies
One of the key strengths of the recent research in this area is the robust combination of experimental designs. Laboratory studies using specific cell lines like SW480 and MCF7, along with animal models, have provided the fine points necessary for understanding these dynamic interactions.
Consider the following table showcasing various study types and their contributions to our current insights:
| Study Type | Key Contributions | Examples |
|---|---|---|
| Human Cell Lines | Demonstrate detailed epigenetic modifications and metabolic shifts at a cellular level | SW480 (colon cancer), MCF7 (breast cancer) |
| Animal Models | Provide a holistic view of tumor progression and microenvironmental influences | APC-mutant mice and other genetically modified models |
| Clinical Cohort Studies | Bridge the gap between laboratory findings and patient outcomes, verifying the relevance of metabolic markers | Large-scale studies using patient data |
| Bioinformatics Analyses | Analyze large datasets from resources like TCGA to identify key epigenetic and metabolic patterns | TCGA databases and public repositories |
These various research methodologies enrich our understanding by allowing us to figure a path through the multi-layered, nerve-racking details that define cancer metabolism and epigenetics. Importantly, integrating these approaches has allowed scientists to discern the potential of dietary interventions and targeted therapies in a burgeoning field that blends modern medicine, alternative treatments, and personalized dietary strategies.
Exploring the Therapeutic Potential of Targeting Metabolic and Epigenetic Pathways
With the recognition that metabolism and epigenetic modifications are interlaced in the progression of cancer, therapeutic strategies have started to focus on these mechanisms. While it might seem intimidating to try and alter such essential processes, the research suggests several promising avenues.
Below are some of the key therapeutic strategies that have emerged:
- Metabolic Inhibitors: Drugs aimed at blocking key enzymes in glycolysis or lipid synthesis can starve tumor cells of requisite energy and building blocks.
- Epigenetic Modulators: Agents that adjust DNA methylation or histone modification patterns could reprogram cancer cells, making them more susceptible to conventional treatment methods.
- Combined Therapy Approaches: Integrating metabolic inhibitors with epigenetic drugs promises not only to disrupt cell growth effectively but also to counteract mechanisms that lead to drug resistance.
- Diet-Based Interventions: Tailoring dietary intake to affect the availability of key metabolic substrates might influence epigenetic markers. For instance, specific nutrients can modify the levels of acetyl-CoA, potentially impacting histone acetylation patterns in tumor cells.
It is important to remember that while these strategies show a lot of potential, the path to their practical implementation is laden with confusing bits and nerve-racking experimental variability. Crafting individualized treatment plans means that doctors must figure a path through the fine points of both a patient’s metabolic profile and the subtle parts of their epigenetic landscape.
Personalized Treatment: Marrying Diet, Metabolism, and Epigenetics
One of the most exciting prospects arising from this dual study of metabolism and epigenetics is the possibility of personalized treatment. Instead of employing broad, one-size-fits-all approaches, clinicians might be able to design strategies that specifically target the metabolic and epigenetic profiles of an individual’s tumor.
This personalized strategy involves several key steps:
- Patient Profiling: Utilizing advanced sequencing techniques to identify metabolic markers and epigenetic patterns unique to a person’s cancer.
- Dietary Adjustments: Creating nutrition plans that limit the supply of substrates which promote undesired epigenetic changes, or conversely, increase levels of metabolites that support beneficial gene expression environments.
- Targeted Drug Therapy: Developing and using drugs specifically designed to counteract the metabolic dependencies of the tumor, or to modify its epigenetic status to re-sensitize it to traditional treatments.
- Monitoring and Adaptability: Continuously tracking a patient’s metabolic and epigenetic markers allows for dynamic treatment adjustments, ensuring that therapy remains effective despite the ever-changing nature of cancer.
When considering the integration of dietary interventions with advanced drug therapies, several fine shades of distinction emerge. The diet does not simply serve as a supportive measure but could be a cornerstone of the therapeutic strategy itself. By carefully managing a patient’s intake of specific nutrients, experts believe that it might be possible to shift the metabolic underpinnings of cancer cells, thereby indirectly tweaking the epigenetic signals that drive tumor growth.
This approach is still in its early stages, and many researchers caution that the journey to fully personalized treatment strategies is full of problems and on edge in terms of clinical implementation. However, the promise of combining nutritional science with molecular oncology offers a super important, innovative avenue for future cancer care.
Challenges and Future Directions: Tackling the Tangled Issues
Despite the breakthrough insights, there remains a long list of nerve-racking challenges that researchers must overcome. Many of the studies conducted so far, while illuminating, also reveal how loaded with issues the field is. The following are some of the key challenges in melding metabolism and epigenetics for cancer treatment:
- Standardizing Experimental Protocols: With studies conducted in varied experimental conditions—from human cell lines to animal studies—the small twists in the data can sometimes lead to conflicting results. Harmonizing methodologies will be key to building reliable treatment protocols.
- Identifying Reliable Biomarkers: Distinguishing which metabolic or epigenetic markers are most predictive of treatment response remains a technical but critical piece in the puzzle.
- Integrating Data Across Disciplines: Merging insights from genomics, metabolomics, and dietary research requires robust bioinformatics tools. The ability to steer through these vast amounts of data is essential to identify actionable targets.
- Clinical Translation: Even with promising laboratory results, turning these findings into effective treatments requires extensive clinical trials. This phase is often on edge due to the numerous variables introduced when moving from a controlled lab setting to diverse patient populations.
To address these challenges adequately, future research must focus on well-designed clinical trials that take into account the tricky parts of metabolic reprogramming, as well as more realistic models of the tumor microenvironment. Collaborative efforts between clinicians, molecular biologists, and nutrition experts will be key to taking a closer look at the hidden complexities of cancer treatment.
The Impact of Bioinformatics and Big Data Analysis
An equally important component in untangling the intertwined mechanisms of metabolism and epigenetic regulation is the role of bioinformatics. With the availability of large datasets from initiatives such as The Cancer Genome Atlas (TCGA), researchers can now get into the nitty-gritty of how cancer develops and progresses on a molecular level.
Advanced computational models help to piece together vast arrays of data—ranging from genetic mutations and epigenetic modifications to metabolite concentrations and patient outcomes. These models are critical in the following ways:
- Data Integration: Merging data from multiple sources can reveal subtle but critical differences in cancer subtypes, helping to tailor treatment protocols.
- Predictive Analytics: Sophisticated algorithms can predict how a tumor might respond to specific metabolic or epigenetic modulations, providing a roadmap for personalized interventions.
- Biomarker Discovery: Uncovering consistent patterns amidst large datasets can help identify new biomarkers that signal treatment efficacy or resistance, guiding future research and therapy.
Bioinformatics serves as the backbone of this evolving field and underscores how essential it is to use data-driven approaches when making decisions about future cancer therapies. With continuous improvements in computational power and algorithms, the prospect of truly personalized treatment plans—tailored to an individual’s unique metabolic and epigenetic landscape—seems not only possible but increasingly accessible.
Integrating Nutritional Strategies for Enhanced Therapeutic Outcomes
One of the most novel aspects of current research is the potential for using nutritional strategies to modify cancer’s metabolic blueprint. Although dietary interventions might seem like a secondary consideration compared to high-tech drug therapies, the influence of food on metabolism makes it a key player in the fight against cancer.
Dietary modifications could help adjust the availability of crucial metabolites and may work in tandem with both metabolic inhibitors and epigenetic modulators. Consider the following points to understand how nutrient-based interventions could be integrated into personalized treatment plans:
- Sugar Intake Management: Since many tumors rely heavily on glycolysis, reducing high-glycemic foods could theoretically slow down the energy supply to tumor cells.
- Fatty Acid Balance: Regulating consumption of certain fats could impact lipid synthesis pathways in cancer cells, potentially reducing the raw materials needed for tumor growth.
- Micronutrient Support: Vitamins and minerals that play roles as cofactors in metabolic reactions might be adjusted to favor anti-tumor epigenetic modifications.
- Dietary Fiber: High fiber diets support beneficial gut microbiota which, in turn, produce metabolites that could positively affect epigenetic markers.
Multiple studies have showcased how even small changes in diet can lead to significant shifts in metabolic pathways. These shifts might not only impede tumor progression but also improve the overall responsiveness to conventional treatments. When combined with targeted drug therapies, nutritional strategies become a must-have tool in the comprehensive management of cancer care.
Patient Stories and the Future of Personalized Medicine
Amid the science and clinical data, real patient stories remind us that the ultimate goal of research is improved quality of life and longer survival for those battling cancer. As scientists and clinicians take a deeper look at the interplay between metabolism and epigenetics, patients can look forward to more tailored approaches that consider not only the genetic makeup of their cancer but also the subtle parts of their overall metabolic health.
Consider the following hypothetical case study:
| Aspect | Traditional Treatment | Personalized Approach |
|---|---|---|
| Gene Expression Analysis | Broad-spectrum chemotherapy with limited targeting | Detailed mapping of epigenetic markers to guide drug selection |
| Dietary Management | General nutritional advice | Customized dietary plan focusing on lowering high-glycemic and unhealthy fat intake |
| Metabolic Profiling | Standard lab tests | Advanced metabolomic analysis to identify metabolic vulnerabilities |
| Therapy Adjustment | Fixed treatment regimen | Adaptive treatment adjustments based on real-time bioinformatics data |
In personalized treatment strategies, the combination of metabolic profiling, detailed epigenetic analysis, and nutrition-based interventions can potentially steer through the nerve-racking maze of cancer care. Patients benefit from treatments that not only target the tumor at a molecular level but also address the broader metabolic context that influences disease progression.
Collaborative Research and the Road Ahead
The path to achieving truly personalized cancer treatment is on a journey filled with both promising discoveries and nerve-racking challenges. One of the more promising aspects lies in the collaborative nature of modern science. With experts in molecular biology, nutrition, alternative medicine, and advanced computational analysis working together, the future of cancer care looks to be both innovative and patient-centric.
Key collaborative areas include:
- Interdisciplinary Teams: Bringing together clinicians, nutritionists, molecular biologists, and data scientists to formulate comprehensive treatment protocols.
- Integrated Data Platforms: Leveraging big data from various sources to quickly adjust therapeutic approaches as new insights come in.
- Patient-Centered Research: Focusing on real-world patient experiences to ensure that the proposed interventions meet practical needs and improve quality of life.
These collaboratives are in a position to tackle both the tangled issues and the subtle details that often complicate standard treatment methods. With such teamwork, the scientific community is better prepared to take a closer look at every component of the metabolic and epigenetic dance in cancer.
Concluding Thoughts: Charting a New Course for Cancer Therapy
The cross-talk between metabolism and epigenetics in cancer is a vivid demonstration of how our understanding of biology continues to evolve. While the interplay is full of problems and loaded with issues, especially when viewed through the lens of traditional treatments, the prospect of personalized medicine offers hope. By carefully managing dietary interventions, employing targeted metabolic and epigenetic therapies, and using bioinformatics to monitor progress, the future of cancer care is set to become more refined and tailored.
There is no denying that the journey is filled with twists and turns—from the initial, nerve-racking measurements in the laboratory to the complex recalibrations required in clinical practice. However, as researchers figure a path through these challenging yet fascinating aspects, a comprehensive approach that integrates modern medicine with innovative nutritional and alternative therapies is emerging.
This evolving picture is not without its setbacks or confusing bits. Yet, each new discovery adds to the body of evidence that a detailed understanding of metabolic reprogramming and epigenetic modifications can lead to super important breakthroughs in the management of cancer. For patients, this means that treatment is moving away from a one-size-fits-all approach toward strategies that are as unique as the individuals facing the disease.
In conclusion, the connection between metabolism and epigenetics in cancer is one of the most exciting frontiers in medical research. As the science continues to slowly untangle these complicated pieces, the potential for more adaptive, individualized cancer therapies becomes clearer. While the challenge of fully integrating these approaches into everyday clinical practice remains on edge, each step forward paves the way for treatments that could fundamentally change the landscape of cancer care.
In this brave new world of personalized treatment, where the interplay of diet, metabolism, and epigenetic fine-tuning can no longer be ignored, it becomes essential to remain open to multi-disciplinary approaches. As studies continue, and as our understanding of the hidden complexities grows deeper, there is a promising future where every patient might receive a treatment plan designed precisely for their own biochemical and genetic makeup.
Ultimately, this integrated approach embodies the spirit of modern medicine—a commitment to improving lives, pushing scientific boundaries, and ensuring that every twist and turn in the journey of cancer treatment is met with determined, thoughtful innovation. By taking a closer look at metabolism and epigenetics, researchers are not only unravelling the nerve-racking details of cancer’s progression; they are laying the groundwork for a future where personalized, patient-centered care is not a pipe dream, but a clinical reality.
While challenges remain, the path ahead is paved with promise. For patients, clinicians, and researchers alike, this burgeoning field offers a beacon of hope—a chance to rewrite the narrative of cancer treatment through the careful integration of nutritional strategies, precise metabolic interventions, and the subtle yet powerful influence of epigenetics.
As we continue to uncover the fine points of these processes, one thing remains clear: a deeper understanding of the metabolic and epigenetic underpinnings of cancer may well be one of the most critical steps toward achieving truly personalized medicine in the 21st century.
Read more about this topic at
Interplay Among Metabolism, Epigenetic Modifications, and …
Interplay between metabolism and epigenetics: a nuclear …