Revolutionizing Hemophilia A Treatment with Tailored Nanolipids
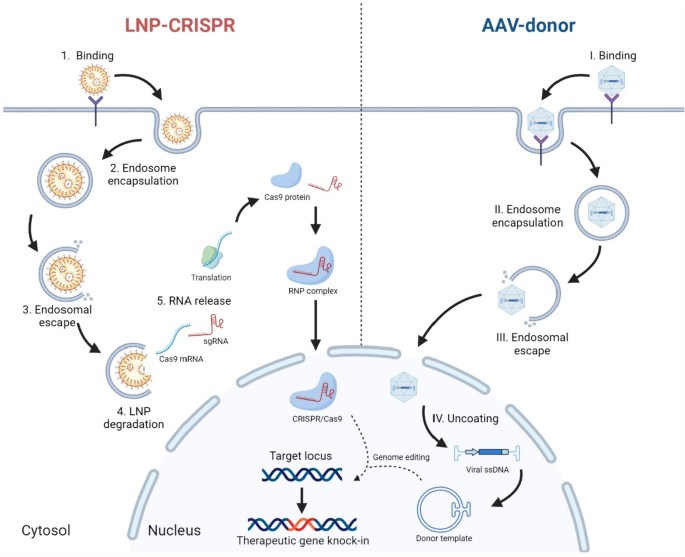
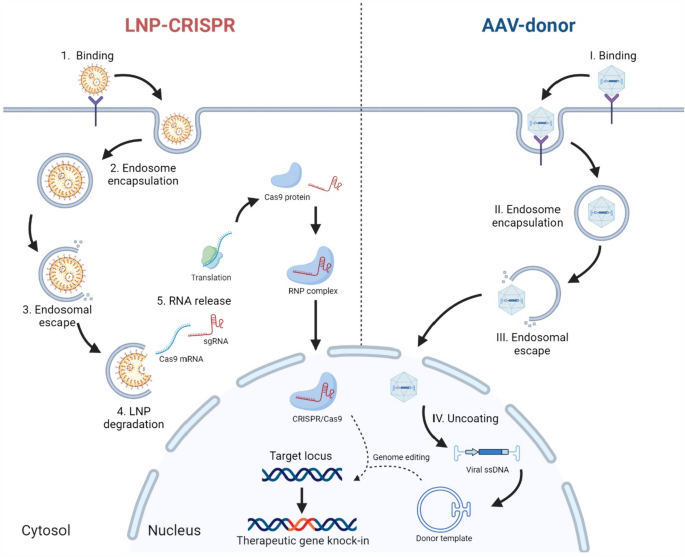
The field of gene therapy has witnessed tremendous advances as researchers continuously work through the tricky parts of delivering genetic material safely and effectively. A recent study exploring biomembrane-inspired lipid nanoparticles (LNPs) for CRISPR-Cas9 delivery in hemophilia A models has sparked a lively debate in the scientific community. This opinion editorial examines the research findings and their potential to reshape how we approach genetic disorders, while also providing insights into the subtle details of LNP design and CRISPR gene editing.
As a professional healthcare journalist, I find it both fascinating and reassuring to see new delivery platforms that address some of the nerve-racking challenges traditionally associated with viral vectors. The study in question reveals that by incorporating natural lipid components such as sphingomyelin and 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine, researchers have been able to boost mRNA uptake in liver cells, enhance endosomal escape, and improve intracellular stability. These advances offer a promising alternative for patients with hemophilia A—a disease where even minor improvements in factor VIII levels can be life-changing.
CRISPR Gene Editing Techniques for Liver Diseases
CRISPR-Cas9 technology has been at the forefront of genetic research for more than a decade, but one of its major challenges has been safely and efficiently delivering the CRISPR components to the target cells. Through the lens of this new study, we see a clear example of how nonviral platforms can be refined to get around the twists and turns of gene therapy.
In traditional CRISPR delivery methods, the use of viral vectors, while effective in some cases, carries risks such as insertional mutagenesis and prolonged nuclease activity. With biomembrane-inspired LNPs, the focus shifts to transient mRNA delivery, thereby limiting the risks associated with permanent genetic modification. This delicate balance of efficiency and safety holds important implications not only for hemophilia A treatment but also for a broader range of liver diseases.
Key Benefits of LNPs in CRISPR Delivery
- Enhanced mRNA uptake in hepatocytes
- Improved endosomal escape and intracellular stability
- Lower off-target activity and systemic toxicity
- Transient expression reducing risks associated with viral methods
These benefits demonstrate that nonviral, lipid-based systems can provide patients with a sophisticated and effective genetic therapy. Researchers observed a 2.3-fold increase in gene editing efficiency in the liver when using optimized formulations compared to standard benchmarks. Such improvements are noteworthy as we continue to tackle those intimidating hurdles in gene-editing delivery systems.
Exploring the Tricky Parts of Lipid Composition and Stability
One of the most intriguing aspects of the study is the detailed analysis of lipid composition. It turns out that maintaining cholesterol at levels between 40% and 50% is critical for ensuring the stability and performance of the targeted gene editing.
By experimenting with different lipid configurations, the research team revealed that using a combination of 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine and sphingomyelin not only increases editing frequency but also ensures that these nanoparticles maintain a favorable distribution within hepatic tissue. The results suggest that even slight differences in lipid makeup can have a dramatic impact on the efficiency of gene correction in liver cells—a testament to the significance of fine-tuning in modern therapeutic development.
Optimizing Lipid Nanoparticle Formulations
To get into the nitty-gritty of optimizing LNP formulations, consider the following key observations from the study:
| Lipid Component | Role in LNP Performance | Observed Effect |
|---|---|---|
| Cholesterol (40%-50%) | Stability enhancer and structural integrity | Optimal editing performance at correct composition |
| 1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine | Facilitates cellular uptake and endosomal escape | Approximately 2-fold increase in editing frequency |
| Sphingomyelin | Improves intracellular mRNA delivery | Enhances hepatocyte uptake |
| Alternative Ceramides (e.g., C18-galactosyl ceramide) | Potential for further optimization | Comparable or better delivery performance |
This table underscores that the secret to success lies in the hidden complexities of lipid selection. Each lipid component is chosen not only for its individual properties but also for how well it interacts with other ingredients to form a robust nanoparticle capable of carrying CRISPR components to target cells.
Balancing Safety, Efficacy, and Immune Response
An essential part of adopting any new medical intervention, particularly in the realm of gene editing, is ensuring that safety is not compromised. In this study, safety assessments of the biomembrane-inspired LNPs showed promising results, with the delivery system maintaining excellent liver targeting and avoiding harmful off-target insertions. Even more reassuring was the absence of any histopathologic evidence of organ injury up to 8 weeks after treatment.
When evaluating immune measures, the researchers noticed that inflammatory cytokine levels, particularly interleukin-6, were dose dependent. However, the levels remained low within efficacious ranges. This indicates that the immune activation observed could be attributed more to the total RNA load rather than the guide RNA itself. Furthermore, flow cytometry analysis illustrated that cell viability remained above 75% at therapeutic RNA concentrations, suggesting that the saturation effects noted are due to the biological capacity of cellular uptake, rather than a sign of overt cytotoxicity.
Balancing Efficacy and Immune Safety
Understanding the balance between a robust therapeutic response and the immune system’s response requires a detailed analysis. Let’s break down the essential considerations:
- Low Off-Target Effects: The CRISPR system modified via LNPs exhibited minimal off-target insertion, reducing concerns about inadvertent genetic modifications.
- Duration of Expression: The transient nature of mRNA delivery means that the exposure to CRISPR nuclease is limited, which can reduce risks such as prolonged unwanted nuclease activity.
- Cell Viability and Cytotoxicity: Maintaining over 75% cell viability at therapeutic doses helps reassure clinicians about the potential for safe human use.
These findings support the idea that fine-tuning and calibrating the delivery system is fundamental to achieving both a therapeutic effect and a tolerable safety profile. For patients with hemophilia A, where liver-directed therapy is essential to restore factor VIII levels, this balance could prove critical for long-term success.
Long-Term Implications for Hemophilia A and Beyond
One of the most groundbreaking results from the study was observed in a mouse model where a single dose of the optimized nanoparticle formulation, termed C7, produced sustained gene correction. The mice showed plasma factor VIII activity levels exceeding 50% of wild-type levels that persisted for more than 12 weeks after treatment. Such durable effects are promising because they suggest a near one-time treatment could potentially result in lasting therapeutic benefits.
For individuals suffering from hemophilia A, even modest increases in factor VIII activity can markedly improve quality of life. This study points toward a future where complex gene editing could transform treatment paradigms, reducing the heavy reliance on constant therapeutic administration—a feat long sought by both clinicians and patients alike.
Extended Benefits Beyond Hemophilia A
While the focus of the research is on hemophilia A, the lessons learned have broader implications for other genetic disorders. Consider the following potential applications:
- Liver-Based Disorders: Conditions such as familial hypercholesterolemia or certain metabolic diseases could benefit from a similar LNP-based CRISPR approach.
- Rare Genetic Diseases: Nonviral delivery systems provide a flexible platform for addressing a range of rare genetic conditions that have so far been difficult to treat.
- Cancer Therapeutics: Improved CRISPR delivery methods might enhance the precision of tumor-targeting therapies, potentially leading to safer regenerative strategies for cancer patients.
These extended benefits reinforce the idea that the advances in nanoparticle engineering are not confined solely to hemophilia A. Instead, they open the door to innovative applications for liver-directed and potentially systemic genome editing interventions. It is a vibrant area for future research that could eventually lead to transformative clinical therapies.
Addressing the Confusing Bits in Nonviral Genome Editing
For those who may find the technical details overwhelming, it is important to break down the confusing bits into more digestible parts. Nonviral delivery methods, such as those based on tailored nanolipids, provide a compelling alternative to viral approaches. The key advantages are centered around safety and transient expression—which significantly reduce the chances of long-term side effects.
However, like any cutting-edge technology, there are complicated pieces that require thorough research and careful optimization. For instance, the precise mixing ratios of lipids, the lipid chemical composition, and the physical properties of the nanoparticle all play super important roles in ensuring that CRISPR components are delivered effectively without triggering off-target immune reactions. Researchers are continuously working to fine-tune these little details to further elevate the safety and robustness of this therapeutic approach.
Simplifying the Process
For a clearer understanding, consider this simplified breakdown of how tailored nanolipids enhance the CRISPR process:
- Designing the Nanoparticle: By choosing specific lipids (such as sphingomyelin and specialized ceramides), scientists create a nanoparticle capable of improved entry into liver cells.
- Ensuring Stability: Maintaining the correct cholesterol levels ensures that the particle remains stable in circulation long enough to reach the target cells.
- Facilitating Endosomal Escape: The specific lipid makeup helps the mRNA escape from cellular compartments, thereby accessing the cell’s machinery for gene repair.
- Minimizing Immune Activation: By keeping the RNA delivery transient and carefully controlled, the nanoparticle minimizes triggering an overwhelming immune response.
This series of steps helps medical professionals figure a path through the maze of delivery systems available for gene editing interventions. It is a considerable leap forward that combines efficiency with a point-by-point focus on safety.
The Role of Storage Stability and Practical Administration
It is not only about achieving high gene-editing efficiency but also about ensuring that the therapeutic system remains practical for real-world use. One significant advantage of the tailored LNP system is its storage stability. For treatments to be viable on a large scale, the formulations must hold up under various storage conditions and during transport, without compromising performance.
The research shows that these biomembrane-inspired LNPs maintain their functional integrity, which is crucial for eventual widespread clinical adoption. They offer a flexible option for liver-directed treatments that could eventually be applied in routine clinical settings. The consistency in delivery performance, along with the scalable production methods, underscores the potential of this technology to overcome some of the nerve-racking practical issues inherent in many modern therapies.
Practical Considerations for Clinicians
If you are a clinician or healthcare provider keeping an eye on the latest advancements, consider these practical points when evaluating tailored LNPs for CRISPR delivery:
- Storage and Handling: The stability of these nanoparticle formulations minimizes the risk of degradation, ensuring that the therapy remains potent until administration.
- Ease of Administration: Single-dose treatments that offer long-lasting effects reduce the treatment burden on patients and healthcare systems alike.
- Side Effect Profile: Lower systemic toxicity and minimal immune activation mean a potentially safer profile compared to traditional viral vectors.
- Scalability and Cost: The production processes for these lipid nanoparticles are evolving, promising a more affordable and scalable solution for gene therapy applications.
These considerations are critical as the field moves toward more precision-based, patient-friendly treatments. The real-world applicability of such technology will depend largely on these additional factors beyond just the laboratory results.
The Future of Nonviral CRISPR Therapies: Challenges and Opportunities
No discussion of emerging medical technologies is complete without addressing the challenges that lie ahead. While the advances in tailored nanolipid design for CRISPR delivery hold great promise, there remain several tricky aspects and tangled issues that the scientific community must work through to ensure that the transition from bench to bedside is smooth and reliable.
Regulatory hurdles, long-term safety assessments, and manufacturing consistency are among the key obstacles that researchers and pharmaceutical companies will need to manage. Additionally, while the current study shows promising results in animal models, further work is needed before these techniques can be broadly implemented in human clinical trials.
Challenges Ahead
Some of the intimidating hurdles that remain include:
- Regulatory Approval: Ensuring that these therapies meet the critical guidelines set forth by health regulatory bodies worldwide.
- Long-Term Safety: Continuous monitoring for any signs of off-target effects or immune-related issues will be essential in long-term studies.
- Scalability of Production: Transitioning from small-scale laboratory experiments to mass production without compromising the subtle lipid compositions represents a significant challenge.
- Cost-Effectiveness: Making sure that these advanced therapies remain affordable once they reach the market.
Addressing these challenges is not a trivial task, but the combined expertise in innovative nanoparticle formulation and CRISPR gene editing suggests that these obstacles are manageable. Continued collaboration between research institutions, clinical professionals, and regulatory agencies is key to turning these promising results into transformative therapies for patients.
Opportunities on the Horizon
Despite the nerve-wracking challenges, the opportunities presented by tailored LNP-based CRISPR therapy are substantial. Here are a few key opportunities to consider:
- Enhanced Therapeutic Options: Patients with hemophilia A and other liver-related genetic disorders might finally have a one-and-done treatment approach, significantly reducing long-term treatment burdens.
- Precision Medicine: This technology paves the way for more precise genome editing that can be tailored to individual patients’ genetic profiles.
- Broader Clinical Applications: The same platform might be adapted for use in treating a wide range of genetic disorders beyond hemophilia A, such as metabolic diseases and certain cancers.
- Reduced Side Effects: The transient nature of mRNA delivery minimizes risks that have long plagued traditional gene therapy approaches, ensuring a safer profile for patients.
The research discussed not only highlights the potential clinical benefits but also encourages a broader conversation about the future of nonviral gene therapy. With each incremental breakthrough in nanoparticle design and CRISPR delivery, we are one step closer to addressing the tangled issues that have so long hindered the field of gene editing.
Comparing Traditional Viral Vectors with Lipid Nanoparticles
For many years, viral vectors were seen as the gold standard for gene delivery due to their natural efficiency in entering cells. However, they come with their own set of intimidating disadvantages, including potential insertional mutagenesis, prolonged nuclease activity, and an increased risk of immune response. In contrast, the tailored LNP approach offers a refreshing alternative with several key advantages.
Let’s compare the two approaches side by side:
| Parameter | Viral Vectors | Lipid Nanoparticles (LNPs) |
|---|---|---|
| Delivery Method | Permanently integrates viral DNA into the host genome | Temporarily delivers mRNA for transient expression |
| Risk Profile | Higher potential for insertional mutagenesis and long-term immune activation | Lower risk due to transient expression and precise targeting |
| Production Complexity | Challenging manufacturing with safety concerns | Greater stability and scalability with improved storage properties |
| Therapeutic Longevity | Potential for long-lasting effects with risk of integration | Sustained therapeutic effects achieved through precise dosing and formulation |
As the table illustrates, while both systems have their pros and cons, the shift toward LNP-based delivery has been driven by the need to mitigate the nerve-racking complications associated with viral methods. This comparison reinforces the narrative that nonviral delivery systems are an increasingly attractive option for clinical applications.
Concluding Thoughts on the Future of CRISPR Delivery
In conclusion, the development of biomembrane-inspired lipid nanoparticles for CRISPR-Cas9 delivery represents a major leap forward in our ability to address genetic disorders such as hemophilia A. By overcoming some of the confusing bits inherent in traditional gene therapy methods, these tailored nanolipids provide a promising, nonviral route to safe and effective genome editing.
The research highlights the importance of fine-tuning each element from lipid composition to dosing protocols, ensuring that the clinical translation of these technologies is both practical and safe. While there remain several intimidating challenges—such as ensuring long-term safety, regulatory approval, and scalable production—the potential rewards are immense.
This breakthrough not only paves the way for more effective treatments for hemophilia A, but it also opens the door for broader applications in treating various liver-based and rare genetic diseases. In an era where precision medicine is increasingly key, the ability to steer through the twists and turns of gene therapy with advanced nanoparticle-based systems could reshape modern therapeutic landscapes.
Final Reflections
From a clinical perspective, the evolution of nonviral CRISPR delivery platforms provides healthcare providers with a powerful new tool in the fight against genetic disorders. The refined balance between robust therapeutic effects and minimized side effects means that tailored LNPs have the potential to become a cornerstone of future gene therapy protocols. As we continue to dig into these promising developments, it is essential for both the scientific and medical communities to keep a close eye on further advancements, regulatory updates, and long-term clinical outcomes.
This opinion editorial underscores the importance of remaining curious and engaged as we witness the convergence of modern medicine and cutting-edge gene editing techniques. The journey from laboratory discovery to routine clinical practice is full of tricky parts, tangled issues, and occasional nerve-wracking moments. However, the steady progress evidenced by innovations in biomembrane-inspired LNPs gives us reason to be optimistic about the near future of personalized genetic therapies.
Looking ahead, I am confident that continued research, open discussion, and interdisciplinary collaboration will eventually lead to the successful implementation of these technologies, ultimately improving patient outcomes and offering hope to those afflicted by hemophilia A and other challenging genetic disorders.
Key Takeaways
- Tailored LNPs have shown a significant improvement in CRISPR-Cas9 gene editing efficiency in liver cells.
- The careful adjustment of lipid compositions, such as the inclusion of sphingomyelin and specific phosphoethanolamines, yields better intracellular delivery and lower toxicity.
- In vivo studies in hemophilia A models demonstrated durable therapeutic corrections, with factor VIII activity restored to clinically relevant levels.
- The nonviral nature and storage stability of these nanoparticles offer promising avenues for safer and more practical gene therapies.
- Challenges remain in scaling production, regulatory approval, and long-term safety—but the potential rewards of these methods are significant.
As we forge ahead, the dialogue between researchers, clinicians, and policy makers will be key to integrating these advanced tools into everyday clinical practice. The progress to date is both encouraging and a testament to the relentless pursuit of innovation in medicine.
Ultimately, it is the collaboration and shared vision of a medical community dedicated to overcoming every intimidating hurdle that will drive these exciting therapies from the research bench to the patient’s bedside. With each carefully optimized nanoparticle, we step closer to a future where genetic disorders are not a life sentence, but rather a challenge that modern medicine can meet head-on with precision and care.
In the evolving world of gene therapy, embracing novel approaches such as tailored lipid nanoparticles ensures that we continue to find new ways to make our way through the tangled issues of modern medicine—bringing tangible hope and potential cures to those who need them most.
Originally Post From https://www.hematologyadvisor.com/news/nanolipids-crispr-delivery-hemophilia-treatment-risk-tailored/
Read more about this topic at
Tailored Nanolipids Advance CRISPR Delivery for …
CRISPR Delivery Methods: Cargo, Vehicles, and Challenges