Durvalumab in Endometrial Cancer: A Closer Look at a Promising Treatment
The treatment landscape for endometrial cancer is evolving, and recent FDA approval of durvalumab in combination with chemotherapy presents both promise and challenges for healthcare providers. In this editorial, we take a closer look at durvalumab’s role in treating advanced or recurrent primary endometrial cancer that is mismatch repair deficient (dMMR). We aim to explore the tricky parts of treatment, the tangled issues of dosing, the confusing bits of side effects management, and the overall impact on patient care.
Understanding the Approval of Durvalumab for Endometrial Cancer
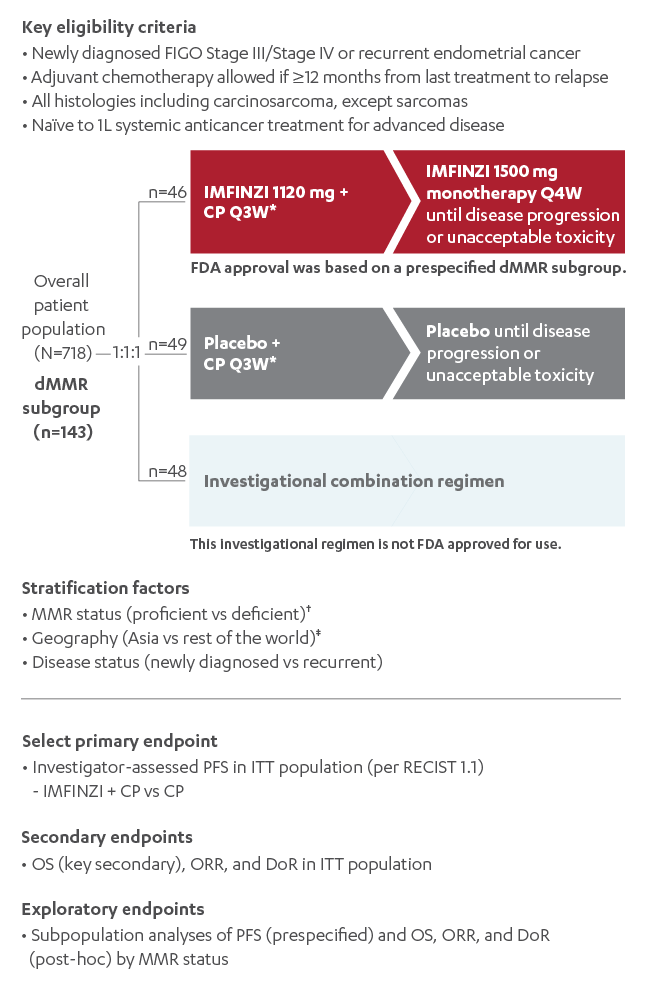
On June 14, 2024, the FDA approved durvalumab (Imfinzi) for adult patients with advanced, recurrent, or primary endometrial cancer with dMMR. This decision came after the randomized, multicenter DUO-E trial (NCT04269200) demonstrated statistically significant improvement in progression-free survival (PFS) in the overall population when durvalumab was administered along with carboplatin and paclitaxel. The trial further revealed that patients with dMMR tumors experienced the greatest benefit.
The approval signifies an essential milestone in oncology treatment, marking the integration of immunotherapy into the standard regimen of chemotherapy. However, as with every breakthrough, there are twisted issues and subtle details that clinicians must account for when incorporating durvalumab into treatment protocols.
Clinical Trial Insights and the Impact on Patient Outcomes
The DUO-E trial provided a wealth of data that clarify the role of durvalumab in endometrial cancer treatment. Patients participating in the trial were divided into groups receiving durvalumab with chemotherapy and a control group receiving placebo with chemotherapy.
The trial’s findings include:
- An improvement in median progression-free survival for patients treated with durvalumab.
- A particularly dramatic benefit in patients with dMMR tumors, where median PFS was not reached compared to 7 months in the placebo group.
- A safety profile that reinforced the importance of careful monitoring of adverse events throughout treatment.
These results highlight the importance of matching treatment strategies to tumor characteristics. The subtle differences in tumor marker status, such as mismatch repair deficiencies, can significantly influence therapy outcomes.
Key Considerations in the Administration of Durvalumab
Treatment with durvalumab is administered via intravenous infusion, and the process is laden with tiny yet key details that healthcare professionals must follow to ensure patient safety. The drug is provided in single-use vials, and there is no oral formulation available. The infusion itself is typically completed over the course of 60 minutes, and unlike other chemotherapy treatments, durvalumab does not require premedication.
It is critical to understand that the dosing of durvalumab is based on body weight. For patients weighing less than 30 kg, dosing is calculated on a milligram per kilogram basis. For those equal to or over 30 kg, fixed doses of 1,120 mg with chemotherapy and 1,500 mg as maintenance are recommended. These weight-based and fixed-dosing strategies require that nurses and other healthcare providers have their patients’ weights checked before each treatment session.
Dosing Protocols: Breaking Down the Process in Simple Steps
The dosing regimen for durvalumab may seem intimidating at first, but it can be understood clearly once the fine points are broken down. Here is a simplified outline:
| Patient Weight | During Chemotherapy (Every 3 Weeks) | Maintenance Phase (Every 4 Weeks) |
|---|---|---|
| < 30 kg | 15 mg/kg (in combination with carboplatin and paclitaxel) | 20 mg/kg |
| ≥ 30 kg | 1,120 mg | 1,500 mg |
This table provides a clear visualization to help staff figure a path through dosing requirements. Although these numbers may seem like just figures, each one represents a critical component of the patient’s overall therapy plan.
How Durvalumab Works: Enhancing the Body’s Immune Response
Durvalumab is a human monoclonal antibody designed to block programmed cell death ligand-1 (PD-L1) from interacting with the programmed cell death receptor (PD-1) and CD80 receptors. This blockade facilitates an anti-tumor immune response by enabling the immune system to recognize and attack cancer cells more effectively.
In simpler terms, durvalumab releases the brakes on the immune system, thus turbocharging the body’s natural defenses. This fine-tuning of immune response is particularly critical in patients whose tumors have already shown resistance to conventional treatments.
Addressing the Tricky Parts of Side Effects Management
While durvalumab has demonstrated significant benefits in the treatment of dMMR endometrial cancer, it is also associated with a range of adverse events (AEs). Handling these side effects effectively is key to ensuring the best possible outcomes for patients. Among the common AEs noted in clinical trials are:
- Peripheral neuropathy
- Musculoskeletal pain
- Nausea and vomiting
- Alopecia
- Fatigue and abdominal pain
- Diarrhea and rash
In addition to these, laboratory abnormalities such as decreased magnesium levels or liver function enzyme increases have also been recorded. Notably, immune-mediated adverse events, such as pneumonitis, endocrinopathies, and severe dermatologic reactions, may occur and require prompt management.
Essential Guidelines for Nurses: Prioritizing Patient Safety
For nurses administering durvalumab, there are several off-putting but essential guidelines to follow. Given the wide variety of side effects and potential immune-mediated complications, patient monitoring during and after the infusion is super important. Here are a few key practices:
- Monitor the patient closely during the infusion, ensuring they remain within eyesight of the nurse’s station.
- Educate patients to alert staff immediately if they experience symptoms such as shortness of breath, rash, or unusual fatigue.
- Maintain strict adherence to infusion protocols, including the proper handling of hazardous drugs.
- Utilize personal protective equipment (PPE) such as gloves, gowns, and eye protection to ensure both patient and staff safety.
With these measures in place, nurses make it easier to get around the potentially nerve-racking aspects of managing infusion-related reactions.
Immune-Mediated Adverse Events: Recognizing the Subtle Details
One of the most challenging areas in the management of durvalumab treatment is the occurrence of immune-mediated adverse events. These immune responses can affect any organ system and are known to be severe—and sometimes even fatal. Recognizing the small distinctions between mild and severe adverse events is crucial.
Immune-Mediated Pneumonitis
Patients with a history of thoracic radiation are particularly susceptible to immune-mediated pneumonitis. Symptoms like a worsening cough, chest pain, or shortness of breath should trigger immediate attention. For grade 1 or 2 pneumonitis, therapy is placed on hold, whereas grades 3 or 4 may lead to permanent discontinuation of treatment. The management of these cases often involves corticosteroids, supplemental oxygen, and in some severe cases, pulmonary rehabilitation.
Immune-Mediated Endocrinopathies
Endocrine disorders such as thyroiditis, hyperthyroidism, hypothyroidism, adrenal insufficiency, and type 1 diabetes can emerge. The approach here generally involves starting hormone replacement therapy for hypothyroidism or instituting appropriate management for hyperthyroidism. Regular monitoring of thyroid function is key, as is keeping an eye out for signs of diabetes that might require insulin management.
Immune-Mediated Dermatologic Reactions
Skin reactions can range from mild rashes to severe conditions like Stevens-Johnson Syndrome (SJS) or toxic epidermal necrolysis (TEN). The management typically involves topical emollients and sometimes corticosteroids. If severe exfoliative dermatitis is identified, treatment protocols may require more aggressive interventions.
Patient Education: Demystifying the Treatment Journey
Patient education is essential when starting treatment with durvalumab. Given the nerve-racking details and twists and turns of immune therapy, patients should be well-informed about:
- The reason behind choosing durvalumab as part of their treatment regimen.
- The mode of administration and frequency of infusions.
- The potential side effects, ranging from common issues like nausea and fatigue to more serious immune-mediated events.
- The necessity of regular laboratory tests before each treatment cycle to monitor complete blood count, metabolic panels, and specific organ functions.
By educating patients thoroughly, healthcare providers can help alleviate the overwhelming uncertainty that often accompanies cancer treatment. This clarity enables patients to better manage and communicate any complications they experience during therapy.
Preparing for Durvalumab: What Every Healthcare Provider Needs to Know
Before the infusion of durvalumab, a series of preparation steps must be followed to ensure safety and effectiveness. Healthcare providers must:
- Verify proper patient identification and history, ensuring that the patient meets all criteria for durvalumab use.
- Confirm laboratory values and review previous imaging studies to monitor treatment progress.
- Inspect the medication and infusion equipment to ensure compliance with safety protocols.
- Educate the patient about what to expect during and after the infusion, including symptoms that warrant immediate attention.
These steps, while seemingly routine, contain many hidden complexities that require healthcare professionals to remain vigilant and proactive throughout the treatment process.
Critical Collaboration: Working Through Potential Side Effects with a Multidisciplinary Team
One of the key aspects of managing immunotherapy, particularly with durvalumab, is the need for a multidisciplinary approach. Physicians, nurses, and specialists such as cardiologists, endocrinologists, pulmonologists, and dermatologists must coordinate to address and manage immune-mediated adverse events effectively.
This collaboration ensures that every subtle detail—from small shifts in laboratory values to slight differences in symptom presentation—is noticed and properly addressed. The teamwork helps create a safety net for the patient, ultimately leading to better outcomes and a smoother treatment course.
Table: Overview of Common Adverse Events and Their Management Strategies
| Adverse Event | Prevalence | Management Strategy |
|---|---|---|
| Peripheral Neuropathy | ≈ 61% | Pain management, supportive care, dosage modifications as needed |
| Musculoskeletal Pain | ≈ 59% | Analgesics, physical therapy, monitoring for progression |
| Nausea/Vomiting | ≈ 59% | Antiemetics, hydration, supportive dietary guidelines |
| Alopecia | ≈ 52% | Counseling, supportive care, use of camouflage techniques |
| Fatigue/Abdominal Pain | 41% / 39% | Rest, pain management, potential treatment adjustment |
| Immune-Mediated Conditions | Variable incidence | Corticosteroids, hormone replacement, specialty referrals |
Overcoming the Overwhelming Aspects of New Treatment Modalities
Introducing a novel treatment such as durvalumab in a clinical setting can appear intimidating at first. With so many moving parts and potential side effects, it is understandable that both patients and providers might feel overwhelmed. However, by breaking down the numerous steps into manageable segments—as we have attempted above—the process becomes more transparent and easier to follow.
For healthcare providers, this means investing time into staying updated with the latest protocols and learning to figure a path through the challenging nuances. For patients, it involves clear communication and education that transforms a complicated treatment regimen into a supported journey toward recovery.
Future Perspectives: Durvalumab’s Place in the Broader Treatment Landscape
As we take a closer look at the utilization of durvalumab for endometrial cancer, it becomes clear that this treatment is part of a larger shift towards personalized medicine. Tailoring therapies to the genetic and molecular makeup of tumors is fast becoming a standard approach in oncology. Although these diagnostic and treatment strategies come with their own set of twisted issues and fine points, they ultimately lead to better outcomes.
The next few years will likely see further integration of immunotherapeutic agents like durvalumab with other targeted therapies. Continued research, including ongoing trials with additional maintenance therapies such as olaparib, will add further layers of nuance to treatment decisions. While the current data for durvalumab is promising, there remains a need to dig into long-term outcomes, overall survival rates, and quality-of-life indices for patients undergoing these therapies.
Practical Tips for Healthcare Providers: Finding Your Path Through Continuous Education
In an era of rapid advancements in oncology, healthcare providers must stay informed about the subtle parts of emerging treatments. Here are a few practical tips:
- Continuous Learning: Regularly attend CME/CE courses and webinars focused on immunotherapy and its management. This will help you stay on top of new developments.
- Interdisciplinary Collaboration: Develop robust communication channels with specialists across various fields to manage complex cases effectively.
- Patient-Centered Communication: Spend dedicated time educating patients on what to expect, emphasizing common symptoms and when to seek help.
- Use of Guidelines: Always refer to updated treatment protocols and guidelines provided by expert committees to ensure evidence-based care.
Managing the Monitoring Process: Tracking the Fine Points of Patient Status
One of the key components of successful durvalumab therapy is vigilant monitoring. This involves periodic laboratory evaluations, imaging studies, and clinical assessments to track the patient’s response to treatment as well as any emerging side effects. The following are essential monitoring parameters:
- Regular blood counts and metabolic panels
- Liver function tests, including alanine aminotransferase and aspartate aminotransferase levels
- Monitoring for signs of immune-mediated organ inflammation
- Assessing for respiratory symptoms that could indicate pneumonitis
- Evaluation of endocrine function, particularly thyroid status
This ongoing monitoring process not only helps in the early identification of adverse events but also ensures that treatment adjustments can be made promptly if necessary. By finding your way through these follow-ups, providers can mitigate risks and optimize patient care outcomes.
Integrating Alternative Therapies: Complementary Approaches in a Comprehensive Plan
While the focus of this editorial is on the integration of durvalumab in endometrial cancer treatment, it is equally important to consider the role of complementary and alternative therapies in supporting patient wellness. Nutrition, physical activity, and stress-management techniques often serve as supportive care measures. Some approaches include:
- Nutritional Counseling: Tailored diet plans that emphasize anti-inflammatory foods can help improve overall health during treatment.
- Physical Exercise: Moderate exercise may reduce fatigue and improve mood, as well as help maintain muscle mass during cancer therapy.
- Mindfulness & Stress Management: Practices such as meditation or yoga can ease the nerve-racking aspects of cancer treatment.
- Integrative Medicine Consultations: These can provide patients with additional perspectives on managing both physical and emotional health throughout their treatment journey.
Integrating these complementary methods into the treatment plan encourages a holistic approach that supports both the physical and mental well-being of patients, ultimately contributing to more balanced and improved outcomes.
Lessons Learned and Future Directions
The incorporation of durvalumab into treatment protocols for dMMR endometrial cancer is a testament to the progress in personalized medicine. The journey through dosing, side effect management, and patient education reveals many of the nerve-racking but manageable aspects of modern cancer treatment. Each clinical trial, patient scenario, and management strategy teaches healthcare providers valuable lessons about meeting the many twisted challenges that these new therapies bring.
Looking ahead, research will continue to explore combinations of immunotherapy with other targeted treatments, ushering in even more refined and customized care plans. Continued dialogue between clinical research teams and frontline healthcare providers will be crucial in refining these protocols, ensuring the best possible outcomes for patient care and quality of life.
Conclusion: Embracing the Future with Knowledge and Compassion
Durvalumab’s approval marks a significant step forward in treating advanced and recurrent endometrial cancer, especially for patients with mismatch repair deficiencies. Through an understanding of the small distinctions in dosing, side effect management, and patient education, healthcare professionals can figure a path toward more effective and safer treatment modalities.
While the complexities of integrating new therapies may seem overwhelming at first glance, breaking down the treatment process into its individual components makes it much more manageable. With clear guidelines, continuous education, and a strong emphasis on patient-centered care, providers can confidently steer through the nerve-racking twists and turns inherent in modern oncology treatment.
Ultimately, the future of cancer care lies in the seamless integration of innovative research with personalized patient management. By leveraging the insights gained from clinical trials like DUO-E and by embracing multidisciplinary collaboration, the medical community is well-equipped to offer more effective and compassionate care for patients battling endometrial cancer.
This editorial serves as an opinion piece meant to encourage ongoing dialogue and continuous learning among healthcare providers. In embracing both the promise and the challenges of immunotherapy, we reinforce our commitment to improving patient outcomes one carefully managed treatment session at a time.
Originally Post From https://www.oncnursingnews.com/view/rx-road-map-durvalumab-for-endometrial-cancer
Read more about this topic at
AGCES: Endometriosis Care & Treatment
Revolutionizing an endometriosis diagnosis: A non- …