Exploring the Role of XBP1s in Breast Cancer Drug Resistance
The landscape of modern oncology is constantly shifting in light of new discoveries, and one of the emerging topics drawing significant attention is the role of XBP1s in mediating resistance to breast cancer treatment. In this editorial, we take a closer look at how XBP1s influences the effectiveness of combination therapies—namely CDK4/6 inhibitors plus endocrine therapy. While the underlying science may at times appear tangled with tricky parts and hidden complexities, we aim to translate the key findings into clear, digestible insights for clinicians, researchers, and informed readers alike.
Recent studies have shown that hormone receptor-positive (HR+) and human epidermal growth factor receptor 2-negative (HER2–) metastatic breast cancer (MBC) patients often face a stubborn challenge: treatment resistance. Despite the promise of CDK4/6 inhibitors combined with endocrine therapy, many patients eventually experience a disappointing loss of response. The discovery that XBP1s, a transcriptionally active spliced variant of XBP1, could be contributing to this cross-resistance has sparked a wave of interest and debate among experts in the field.
Understanding XBP1s in the Context of HR+/HER2– Breast Cancer
Breast cancer, particularly the HR+/HER2– subtype, is notorious for its tricky treatment issues. XBP1, which becomes activated into its spliced form XBP1s, is prominent in this type of cancer and appears to play a significant role in driving cell proliferation and facilitating the cell cycle’s G1/S transition. In simple terms, XBP1s acts as a catalyst that not only speeds up tumor growth but also hampers the effectiveness of current therapeutic interventions.
This revelation underscores a critical concern for oncologists: while the combination treatment of CDK4/6 inhibitors with endocrine therapy has been a game changer, its long-term success is jeopardized when XBP1s steps into play. The molecule essentially creates a survival advantage for the cancer cells, helping them figure a path around treatments that are supposed to suppress tumor growth.
Key Findings: How XBP1s Drives Resistance
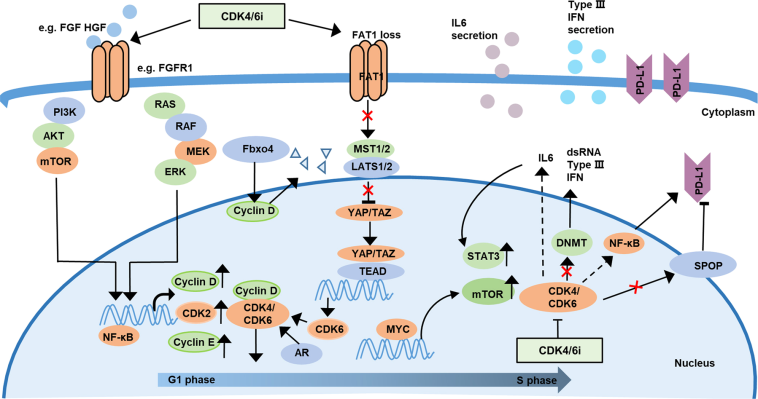
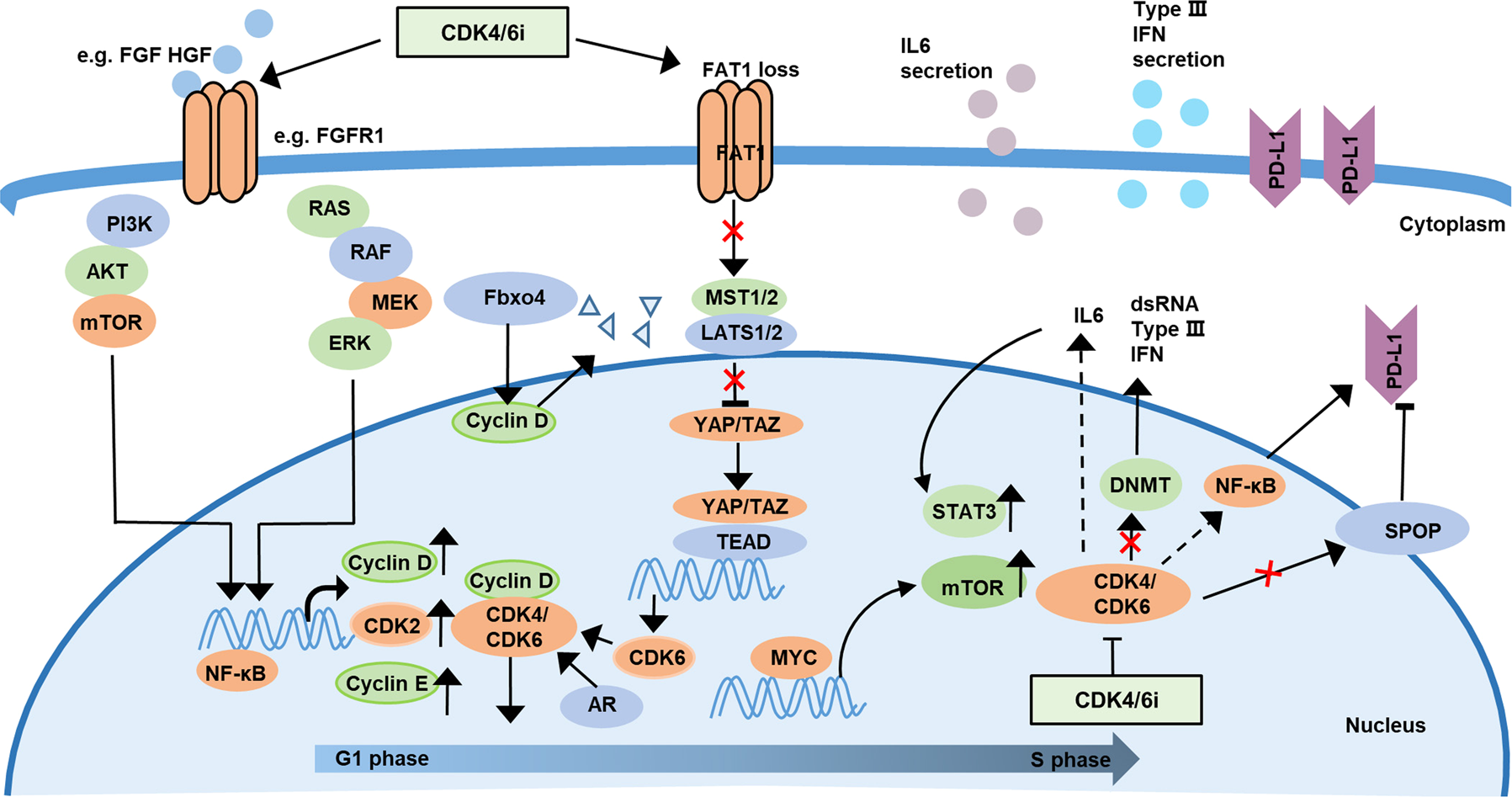
The recent study published in Advanced Science has opened up several avenues for understanding the mechanisms behind resistance. Among the main revelations is that XBP1s can activate downstream targets through the transcriptional activation of SND1. This process eventually stimulates the E2F1 pathway—a key driver of cell cycle progression and proliferation. In essence, when XBP1s is overexpressed, it can set off a chain reaction that makes tumor cells less sensitive to the effects of both endocrine therapy and CDK4/6 inhibitors.
This activation is especially problematic for clinicians because it implies that even when patients are on a well-established combination therapy, the survival mechanisms instigated by XBP1s can undermine therapeutic efficacy. Not only does this make treatment more nerve-racking, but it also raises important questions about how to manage and eventually overcome these complications in a clinical setting.
Digging Into the E2F1 Pathway: The Impact on Cancer Cell Growth
One of the critical pathways implicated in this resistance is the E2F1 pathway. E2F1 is a crucial transcription factor that orchestrates a variety of cell cycle processes. When XBP1s activates this pathway—specifically through the upregulation of SND1—it results in an unabated cell cycle progression, particularly during the G1/S transition. The result is an overall increase in tumor cell proliferation, making the cancer not only more aggressive but also more resilient in the face of treatment.
Even though the notion of cell cycle regulation may have some complicated pieces, its fundamental role in cancer progression is well recognized. Understanding how finely tuned elements like the E2F1 pathway contribute to treatment resistance can help researchers identify vulnerable targets, where intervention might reverse or at least mitigate these problematic outcomes.
Overcoming Resistance: Combining Pharmacological and Epigenetic Approaches
Confronted with the challenge posed by XBP1s, researchers have begun to explore additional strategies to counteract its effects. One promising approach involves the use of both pharmacological inhibitors and epigenetic interventions designed to specifically reduce XBP1s expression. In experimental models, agents like 4µ8C have been shown to not only inhibit the production of XBP1s but also to enhance the sensitivity of cancer cells to the primary combination therapy.
This dual approach is reminiscent of how chefs sometimes tackle a difficult recipe by not only adding a new ingredient but also tweaking the cooking method to overcome the nerve-racking obstacles. By targeting the fine details of XBP1s regulation, researchers are forging new pathways to improve outcomes for patients who have limited options once resistance sets in.
Clinical Implications: What This Means for Patient Care
From a clinical perspective, the implications of these studies are both significant and timely. The identification of XBP1s as a potential predictor of poor progression-free survival in HR+/HER2– MBC patients adds another layer of complexity to treatment planning. It means that oncologists may eventually have to rely on biomarkers like XBP1s to decide which patients will benefit the most—or the least—from the current standard of care.
Imagine a scenario where a simple test indicates that a patient’s tumor expresses high levels of XBP1s. With this information in hand, clinicians could decide to implement an additional intervention aimed at reducing XBP1s activity, potentially steering patients away from a treatment path that might be less effective. Such precision medicine could also be achieved by combining the traditional CDK4/6 inhibitors and endocrine therapy with targeted epigenetic drugs. This multi-pronged strategy may provide a comprehensive way to counteract the malignant survival tactics employed by the cancer cells.
Detailed Table: Key Components of XBP1s-Related Drug Resistance
| Component | Role/Function | Impact on Treatment |
|---|---|---|
| XBP1s | Transcriptionally active spliced variant; promotes cell proliferation | Enhances tumor survival, reducing efficacy of CDK4/6 inhibitors plus endocrine therapy |
| SND1 | Transcriptional activator downstream of XBP1s | Upregulates the E2F1 pathway, fostering a pro-survival environment |
| E2F1 Pathway | Key driver of cell cycle and proliferation | Accelerates G1/S transition, contributing to unchecked tumor growth |
| Pharmacological Inhibitors (e.g., 4µ8C) | Block the expression or function of XBP1s | Potential to restore sensitivity to combination treatment |
This table simplifies the intertwined factors that contribute to drug resistance mediated by XBP1s, illustrating the network of effects that complicate the targeting of breast cancer. The interplay between XBP1s, SND1, and E2F1 creates a scenario loaded with issues that demand a strategic, multi-targeted response.
The High Stakes of Treatment Resistance: A Closer Look at Patient Outcomes
Treatment resistance is not just a laboratory observation—it has real-world consequences for patients battling metastatic breast cancer. For these individuals, facing a resistant tumor can be akin to being caught in a maze of confusing bits and intimidating obstacles. Over time, patients who initially respond well to therapy begin to experience disease progression, ultimately undermining the quality of life and survival outcomes.
In today’s clinical practice, where every day counts, the early identification of resistance patterns is essential. The association between high XBP1s expression and unfavorable clinical responses calls for more proactive measures in patient management. Oncologists might soon find that integrating biomarker testing into routine practice is a must-have component of a truly personalized treatment plan.
Patient-Derived Organoids: Advancing the Study of XBP1s and Drug Resistance
In the realm of experimental medicine, patient-derived organoids have emerged as a powerful tool for modeling disease. These miniaturized, three-dimensional structures replicate many of the fine shades of a patient’s tumor architecture and microenvironment. Researchers have turned to organoids to validate the role of XBP1s in promoting a pro-survival effect against CDK4/6 inhibitors and endocrine therapy.
This innovative approach allows the scientific community to take a closer look at the small distinctions in how direct targeting of XBP1s might restore drug sensitivity. By experimenting on organoids, scientists can better predict how interventions that reduce XBP1s expression will influence the overall behavior of the tumor, thus paving the way for tailored therapies that specifically address the individual patient’s condition.
Strategies for Reversing Cross-Resistance: Combining Traditional and Novel Therapeutic Approaches
The realization that XBP1s could be a key driver of resistance has spurred the development of several therapeutic strategies aimed at reversing this phenomenon. Instead of relying solely on established treatment regimens, a new direction is emerging that combines routine drugs with innovative agents targeting XBP1s.
A multifaceted strategy might include:
- A dual-drug approach where CDK4/6 inhibitors and endocrine therapy are used alongside agents that specifically block XBP1s.
- The utilization of epigenetic modifiers to silence the expression of XBP1s, thereby reducing its downstream effects.
- A personalized medicine framework where biomarker testing informs the selection and combination of therapeutic agents tailored to each patient’s unique profile.
This approach not only aims to clear the tangle of issues surrounding treatment resistance but also provides a blueprint for future combination strategies that could yield better outcomes and improved progression-free survival for patients with HR+/HER2– MBC.
Addressing the Tricky Parts of Implementing Biomarker-Guided Therapy
While the potential benefits of utilizing biomarkers such as XBP1s in treatment planning are clear, there are several tangled issues that need careful consideration. Biomarker-guided therapy requires robust and reliable testing methods. There needs to be a standardized protocol to measure the levels of XBP1s in tumor tissues—a step that is easier said than done.
Some of the challenges include:
- Test Sensitivity and Specificity: Ensuring that the tests can accurately distinguish between subtle differences in XBP1s expression levels.
- Cost Considerations: The expense of biomarker testing could be a barrier, especially in regions with limited healthcare resources.
- Integration into Clinical Workflow: Adding new testing protocols might temporarily overwhelm clinical staff until streamlined processes are established.
Despite these nerve-wracking hurdles, many experts believe that the benefits of a targeted, precision medicine approach will eventually outweigh the initial challenges. After all, improving patient outcomes through targeted interventions remains a must-have goal in modern oncology.
Exploring the Hidden Complexities: Epigenetic Regulation and Its Therapeutic Potential
Epigenetics, the study of heritable changes in gene expression without altering the DNA sequence, is opening up new horizons in cancer therapy. Recent findings suggest that the expression of XBP1s can be influenced by epigenetic modifications. This adds another layer of nuance to the drug resistance riddle.
By diving into the epigenetic landscape, clinicians and researchers are beginning to identify specific modifications that either promote or suppress the production of XBP1s. Targeting these modifications offers a promising strategy to interrupt the malignant cycle of resistance. The integration of epigenetic drugs with traditional treatment regimens creates a scenario where cancer cells may be stripped of their survival tactics—potentially restoring the power of CDK4/6 inhibitors and endocrine therapy.
For instance, epigenetic drugs that modify histones or DNA methylation patterns have shown promise in preclinical studies. These findings are creating optimism that unlocking the hidden complexities of epigenetic regulation may lead to a breakthrough in overcoming treatment resistance.
Lessons From the Lab: Translating Bench Research to Bedside Benefits
The journey from laboratory discoveries to clinical application is often a long and winding road filled with both promising breakthroughs and daunting setbacks. The research on XBP1s and its role in drug resistance is no exception. While laboratory findings have provided valuable insights into the underlying mechanisms of resistance, there remains a need to translate these results into practical, patient-centered treatments.
One important step in this translation is the validation of research findings using patient-derived models, such as organoids. These models help bridge the gap between the controlled environment of the lab and the unpredictable nature of human disease. They allow clinicians to test the effectiveness of new treatment combinations in a setting that closely mimics the patient’s own tumor, thereby providing a more accurate prediction of clinical outcomes.
In my opinion, the biggest takeaway is that even the smallest details—those fine points in molecular signaling—can have a dramatic influence on treatment success. Working through these issues requires a concerted effort from researchers, clinicians, and policy makers alike to ensure that promising therapies do not languish on the shelf but are swiftly and safely brought to the bedside.
Future Perspectives: Tailoring Treatments to Outsmart Cancer
Looking ahead, the need for personalized medicine in breast cancer care has never been more apparent. The identification of molecular drivers like XBP1s paves the way for treatments that tailor therapy to the individual, addressing not just the tumor’s visible characteristics but also its hidden survival strategies. Personalized treatment plans, built on the foundation of precise biomarker data, could potentially transform the standard of care for HR+/HER2– MBC patients.
Future research should focus on several key areas:
- Validation of Biomarkers: Conducting larger clinical studies to validate XBP1s as a reliable predictor of treatment response.
- Combination Therapy Trials: Designing and executing clinical trials that test the efficacy of adding epigenetic modifiers or pharmacological inhibitors of XBP1s to standard treatments.
- Mechanistic Studies: Further investigating how XBP1s interacts with other molecular pathways to build a more complete picture of its role in drug resistance.
- Cost-Effective Testing: Working towards the development of affordable and accurate tests for assessing XBP1s levels in clinical settings.
These steps are critical in creating a future where each patient’s treatment regimen is as unique as their own genetic and epigenetic profile—a future where healthcare providers not only treat the disease but also overcome the complicated pieces that render some therapies less effective over time.
Insights on Balancing Treatment Efficacy and Resistance
As we continue our journey towards more effective cancer therapies, it becomes clear that there is no silver bullet. Instead, the solution lies in balancing the power of existing treatments with innovative strategies designed to circumvent the obstacles posed by molecules like XBP1s. The interplay between effective cell cycle arrest by CDK4/6 inhibitors and the pro-survival signals triggered by XBP1s illustrates the delicate dance between treatment efficacy and resistance.
It is essential for researchers to keep in mind that while targeting one pathway might produce immediate results, cancer cells are notorious for their ability to find an alternative route to survival. This makes the continuous review and adaptation of treatment strategies super important. The integration of novel agents should not be seen as a replacement for existing therapies but rather as an enhancement that works in tandem to ensure that cancer cells are deprived of every possible survival mechanism.
Managing Your Way Through Treatment Decisions: A Clinician’s Perspective
For clinicians, the decision-making process is riddled with tension as they strive to find the right combination of therapies that will yield the best outcomes for their patients. The addition of biomarkers like XBP1s to this decision-making toolkit represents a double-edged sword: it offers the promise of more tailored treatment, but also introduces another variable into an already nerve-wracking equation.
Clinicians must balance several important considerations:
- Patient-Specific Factors: Age, comorbidities, and overall health can influence how a patient responds to therapy.
- Biomarker Expression Levels: Levels of XBP1s and other markers can guide the choice of adding specific inhibitors or modifying doses.
- Treatment History: Understanding how a patient has responded to previous treatments can help predict future success or failure.
- Safety and Tolerability: Ensuring that the chosen treatment regimen does not introduce overwhelming side effects is a constant priority.
By taking a comprehensive look at these factors, clinicians can piece together a treatment approach that not only targets the cancer effectively but also minimizes the risk of allowing the tumor to get around the therapy’s intended effects.
Integrating Lifestyle and Supportive Measures in the Era of Targeted Therapy
While the scientific focus on molecules such as XBP1s is crucial, it is equally important to remember that medical treatment does not operate in a vacuum. Often, patient outcomes can be significantly enhanced when targeted therapies are integrated with supportive measures like proper nutrition, stress management, and physical fitness. These factors, sometimes seen as the small twists that define the overall well-being of cancer patients, can make a substantial difference in how patients tolerate and respond to treatment.
For many patients, the journey through cancer treatment is laden with both physical and emotional challenges. Incorporating complementary therapies—such as dietary adjustments rich in antioxidants and anti-inflammatory foods, personalized exercise programs, and mental health support—can provide a holistic approach that not only improves quality of life but may also enhance treatment efficacy.
Ultimately, a well-rounded approach to cancer care helps address the nerve-racking aspects of diagnosis and treatment, supporting patients in managing their path through each stage of their journey.
Addressing the Unresolved Challenges: A Call for Multidisciplinary Collaboration
The battle against breast cancer is multifaceted and loaded with problems that span laboratory research, clinical practice, and patient care. It is clear that overcoming resistance mechanisms like those driven by XBP1s requires a collaborative effort that bridges these arenas. Researchers, clinicians, and healthcare policymakers must work together to:
- Promote Interdisciplinary Research: Bringing together experts in oncology, molecular biology, epigenetics, and bioinformatics to piece together the small details that underlie treatment resistance.
- Streamline Clinical Trials: Designing trials that incorporate biomarker assessments early in the patient selection process so that future therapies are based on strong, predictive evidence.
- Educate and Empower Clinicians: Providing ongoing training regarding the interpretation of biomarker data and the latest insights into mechanisms of drug resistance.
- Engage Patients: Ensuring that patients are fully informed about their treatment options, potential side effects, and the rationale behind tailored therapeutic approaches.
This multidisciplinary approach is not merely an academic exercise but a practical necessity to ensure that the advances in molecular research translate into meaningful, lifesaving outcomes for patients.
Concluding Thoughts: A Future of Personalized and Adaptive Cancer Care
In conclusion, the discovery of XBP1s as a mediator of cross-resistance in HR+/HER2– breast cancer treatment represents both a challenging twist in the treatment narrative and a promising opportunity for innovation. The evidence suggests that by taking a closer look at this molecule and its role in activating the E2F1 pathway, we have the potential to re-engineer current treatment protocols and advance toward personalized medicine.
The path forward is filled with both confusing bits and subtle parts that require us to get into the deep molecular underpinnings of cancer. However, by adopting a more nuanced and precise treatment strategy—one that incorporates biomarkers like XBP1s, combines multiple therapeutic agents, and integrates supportive lifestyle measures—we can better manage your way through the tangled issues of drug resistance.
It is an off-putting reality that cancer cells are adept at finding alternative ways to survive when confronted with standard treatments. Yet, the excitement in the research community today is palpable. Innovations in both pharmacological and epigenetic strategies hold the promise of not just slowing down the progression of breast cancer, but perhaps even reversing its course in patients who have long faced the scary prospect of treatment failure.
While there remains a good deal of work to be done, the dialogue between laboratory research and clinical practice has never been more important. As we steer through the twists and turns of modern cancer therapy, collaboration and adaptability will be key to translating these findings into improved patient outcomes.
Looking ahead, it is critical that we continue to support and fund research that aims to dissect the complicated pieces of cancer resistance, while simultaneously refining our clinical practices to incorporate these insights. Only then can we hope to develop treatment regimens that are both highly effective and personalized to the unique challenges faced by each patient.
In the final analysis, the battle against breast cancer is a multi-front war—one that demands an integrated approach spanning molecular insights, innovative drug development, and compassionate patient care. By keeping a keen eye on biomarkers like XBP1s and embracing the full spectrum of therapeutic strategies, we are taking important strides toward a future where every breast cancer patient can receive the super important, tailored treatment they deserve.
This is an evolving story, one where the careful piecing together of research findings, clinical experience, and patient insights will guide us toward overcoming the nerve-racking obstacles of drug resistance. The days ahead promise exciting developments as we continue to fine-tune our strategies and move closer to the goal of truly personalized cancer care.
Ultimately, let us remain hopeful and determined. Every small victory in understanding and combating the survival strategies of cancer cells brings us one step closer to a world where treatment resistance is no longer a full-of-problems roadblock, but a challenge that we have learned to outsmart with innovation, precision, and relentless collaboration.
Originally Post From https://pubmed.ncbi.nlm.nih.gov/40940685/
Read more about this topic at
Targeting the IRE1-XBP1 axis to overcome endocrine …
Cancer cell-intrinsic XBP1 drives immunosuppressive …