The Promise of Tumor-Specific Prodrug Strategies in Modern Cancer Therapy
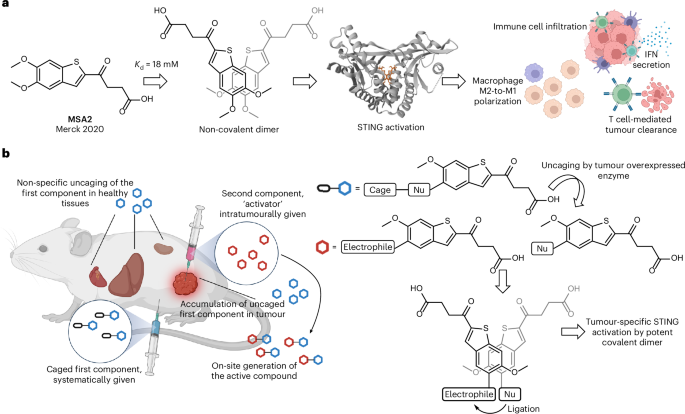
The fight against cancer requires ideas that are both innovative and practical. One such idea is the use of tumor-specific prodrug systems that aim to selectively activate a therapeutic agent only in the tumor microenvironment (TME). In recent years, researchers have made great strides toward harnessing the body’s innate immune mechanisms to battle malignancies. An example of this is a two-component prodrug designed to generate a STING agonist specifically within tumors. This editorial takes a closer look at this emerging technology, discusses its potential benefits, and weighs the challenges and opportunities that lie ahead.
Understanding the Two-Component Prodrug Approach
The concept behind a two-component prodrug system is simple yet ingenious: instead of administering a highly active drug that can affect both healthy and cancerous tissues, two benign precursors are given. These individually harmless compounds interact only in the tumor—where conditions are unique—to generate a potent STING agonist that stimulates an antitumor immune response.
This approach is particularly attractive because it promises to minimize off-target toxicities. It takes advantage of two key factors:
- Enzyme overexpression in the TME (for instance, β-glucuronidase), which serves as a trigger.
- The chemically tailored reactivity between a nucleophile and an electrophile that leads to covalent dimer formation.
STING Pathway Activation: The Immune System’s Alarm Clock
The stimulator of interferon genes (STING) pathway is central to the innate immune response. It acts as an alarm clock by alerting the system when there is trouble—in the form of misplaced DNA or other signs of cellular distress. Activation of this pathway results in the production of type I interferons and other proinflammatory cytokines, cushioning the way for robust immune responses against tumors.
While this pathway is full of promise for cancer therapy, its direct activation in non-cancerous tissues has been linked to unwanted side effects. Such outcomes make it critical to ensure that the powerful signals are turned on only at the tumor site. The new two-component prodrug strategy works by ensuring that the active compound is essentially synthesized on-site, thereby avoiding the widespread delivery that could trigger the immune cascade throughout the body.
How the Prodrug System Works: Getting into the Nitty-Gritty
Understanding this approach means taking a closer look at both the chemistry and biology behind the reaction. Here, two specially modified compounds derived from a parent molecule are used:
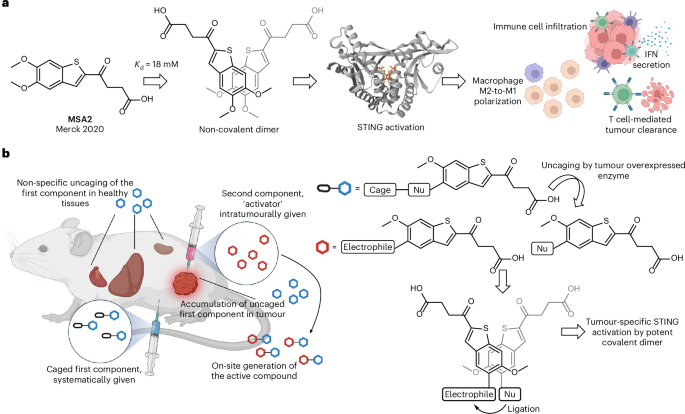
- A nucleophilic component that is caged by a self-immolative β-glucuronide moiety. This design ensures that the nucleophile remains inactive until cleaved by β-glucuronidase—a molecule known to be overexpressed in many cancers.
- An electrophilic component delivered directly into the tumor through intratumoral injection. Its chemical modification renders it inert until it meets its nucleophilic partner.
When these components meet in the tumor, they react under mild, physiologically relevant conditions. The bond-forming process leads to a covalent dimer – a STING agonist that is far more potent than the individual parts. Researchers have observed that even low micromolar concentrations are sufficient for the reaction to proceed effectively under conditions that mimic the human body.
Key Steps in the Reaction Process
The chemical reaction can be viewed as a series of steps, each with its own set of tricky parts and subtle details. Here are the essential stages:
- Enzyme-Triggered Decaging: The enzyme β-glucuronidase, abundant in tumors, removes the protective group from the nucleophilic component. This decaging is a critical, regulated step, ensuring that the reactive moiety is only released where it is needed.
- Proximity and Self-Assembly: The two modified molecules are designed to dimerize through stacking interactions. Such non-covalent engagements help bring the reactive groups into close proximity, accelerating the covalent bond formation.
- Covalent Bond Formation: With the reactive species properly aligned, a covalent bond forms between the nucleophile and the electrophile. The resulting dimer demonstrates submicromolar potency in cell-based assays, marking it as an extremely active STING agonist.
Benefits and Opportunities for Clinical Applications
This prodrug system offers several enticing opportunities for clinical application. By focusing on tumor-specific activation, it aims to overcome some of the major hurdles of current cancer treatments:
- Reduced Systemic Toxicity: Because the active drug is generated only at the tumor site, healthy tissues are spared exposure to a potent immunostimulant.
- Precision in Immune Activation: The localized synthesis of the STING agonist ensures that the immune system’s alarm is sounded only where it is needed. This targeted activation could lead to an improved therapeutic index.
- Potential for Combination Therapy: Given its mechanism, this approach could be combined with other cancer treatments such as checkpoint inhibitors, immunomodulatory agents, or even conventional chemotherapeutics, paving the way for multifaceted treatment plans.
Overcoming the Tricky Parts of Tumor Microenvironment Specificity
Achieving tumor-specific activation requires practitioners to get into the trickier aspects of the tumor microenvironment—a setting that is, at times, full of problems and confusing bits. One challenge is ensuring that enzymes such as β-glucuronidase are present at sufficiently high levels in the TME compared to normal tissues.
Some of the tangled issues include:
- Heterogeneity of Tumor Enzyme Expression: Tumor tissues vary widely in their enzyme profiles. Not every tumor may produce enough β-glucuronidase for effective decaging. This means that patient selection and accurate diagnostic measures become essential.
- Stability in Systemic Circulation: The individual precursor compounds need to remain intact until they reach the tumor site. Researchers have observed that the caged nucleophile shows robust stability in human plasma, but ensuring this under real-life conditions is always a nerve-racking challenge.
- Optimal Dosing and Administration Routes: While one component is given systemically and the other directly into the tumor, balancing the doses to maximize on-site reaction without causing systemic side effects is a delicate task. In animal models, intratumoral injections have been used, but adapting such a method for human patients might prove intimidating.
Diving Into the Chemistry: Covalent Dimerization and Its Advantages
At the heart of this prodrug strategy lies the science of covalent dimerization. The reaction between the modified nucleophilic and electrophilic components is both swift and reliable—a vital consideration when designing therapies that must function in the chaotic milieu of cancer.
One of the less obvious but critical aspects of the approach is the reliance on self-assembling interactions. Before the covalent bond can form, the molecules must first arrange themselves in just the right way. This is no small feat given the many twists and turns of molecular behavior. Studies have shown that the intrinsic reactivity of modified electrophiles can be greatly enhanced when they are drawn together through non-covalent stacking interactions.
Some of the key fine points in this process include:
- Enhanced Reaction Rates: The non-covalent pairing of the two molecules can accelerate the reaction by several orders of magnitude compared to what would be expected from a traditional bimolecular interaction in solution.
- Selectivity Under Complex Conditions: Even in the presence of competing agents such as glutathione, the reaction between the prodrug components remains highly selective. This is crucial in ensuring that the active compound is produced only in the intended location.
- Potency Improvements: The end product—the covalent dimer—has been shown to activate the STING pathway at extremely low concentrations, offering a significant improvement in potency compared to the parent molecule.
Navigating the Tumor Microenvironment: Enzyme-Triggered Activation in Action
One of the most interesting aspects of the two-component prodrug system is how it takes advantage of the unique enzyme landscape of tumors. In many types of solid tumors, areas of necrosis and hypoxia lead to the release of enzymes like β-glucuronidase. Because this enzyme is less active in healthy tissues, it can serve as an effective trigger, ensuring that the drug only becomes active in the right places.
Working through the effect of β-glucuronidase involves a few subtle requirements:
- Substrate Specificity: The caged nucleophile is carefully designed so that it is only uncaged by β-glucuronidase. This specificity helps avoid accidental activation in normal organs.
- Rapid Enzymatic Conversion: Once the enzyme is present, the release of the active nucleophile is rapid, allowing the subsequent reaction with the electrophilic partner to occur almost immediately.
- Maintaining Stability Until Activation: The design ensures that the caged form does not release the active nucleophile prematurely, which is key to preventing unintended systemic immune activation.
Real-World Implications: Animal Models, Imaging, and Beyond
Recent studies using animal models such as zebrafish xenografts and mouse tumor models have provided promising proof-of-concept evidence for the two-component prodrug strategy. These models have shown that when the prodrug components are administered correctly, the active STING agonist is produced almost exclusively within the tumor.
Some of the important observations include:
- Localized Activation in Zebrafish Models: Zebrafish xenograft experiments have demonstrated that, with the help of additional β-glucuronidase, the prodrug can induce apoptosis and robust immune activation at the tumor site. The imaging techniques enabled researchers to see increased levels of active caspase markers and immune cell infiltration.
- Mouse Tumor Model Success: In syngeneic mouse models, the targeted formation of the active dimer led to slowed tumor growth and improved survival. Mass spectrometry confirmed that the active STING agonist remained at high levels in the tumor, with minimal leakage into healthy tissues.
These findings underscore the potential of the approach in real-world conditions. They suggest that, with careful refinement, the treatment could become a key component in the future of targeted immunotherapy.
Charting the Future: Combining with Other Therapies
The selective nature of the prodrug system opens the door for combining this therapy with other treatment modalities. In modern cancer care, combination therapies are seen as a must-have strategy. Here are a few ways in which the two-component prodrug approach could be integrated:
- Checkpoint Inhibitors: Pairing the tumor-specific STING agonist with immune checkpoint blockade could help overcome the immune-suppressive conditions that often accompany tumors.
- Chemotherapeutics: By using the prodrug system alongside conventional chemotherapeutic agents, clinicians may potentially lower the dose of toxic drugs while maximizing therapeutic benefits.
- Radiation Therapy: Radiation not only directly damages tumor cells but can also alter the TME. The changes in enzyme expression following radiation might further enhance the selective activation of the prodrug, thereby creating a synergistic effect.
Table: Advantages of Tumor-Specific Prodrug Approaches
| Aspect | Benefit | Challenges |
|---|---|---|
| Selective Activation | Ensures that the active drug is produced only at the tumor site | Requires high and specific enzyme expression levels |
| Minimized Systemic Toxicity | Reduces damage to healthy tissues | Stability of precursors in circulation must be ensured |
| Enhanced Potency | The covalent dimer formed demonstrates submicromolar activity | Optimizing dosing and reaction kinetics is essential |
| Compatibility with Combination Therapy | Can be paired with other targets or treatments | Integrating different therapies requires careful coordination |
Addressing the Overwhelming Challenges: Balancing Safety and Efficacy
Despite its many promising facets, the tumor-specific prodrug strategy comes with its own set of tricky parts and nerve-racking challenges. Some of the main issues that need further attention include:
- Dose Optimization: Determining the right balance of the two components can be intimidating. It is essential to ensure that sufficient drug is generated at the tumor site while avoiding systemic activation that could lead to side effects.
- Variability Among Patients: The enzyme levels and the specific composition of the TME can vary widely among patients. This variation means that the approach might need to be personalized, adding another layer of complexity to treatment planning.
- Delivery Methods: The current method of intratumoral administration works in preclinical models, but translating this to human patients may require inventive delivery platforms that allow for non-invasive or minimally invasive application.
- Potential Immunogenicity: Even with localized activation, there is a risk that the rapid and strong immune effect could trigger unintended inflammatory reactions. Ensuring a controlled immune engagement is key to long-term success.
Exploring the Fine Points of Chemical and Biological Interactions
Taking a closer look at the chemical reaction between the modified molecules reveals several subtle details worth highlighting. The two components of the prodrug system are designed to interact through predictable chemistry that benefits strongly from the proximity induced by non-covalent stacking. This is crucial for several reasons:
- Reaction Efficiency: The co-localization of the nucleophilic and electrophilic groups results in faster reaction kinetics compared with similar reactions in a less structured environment. This efficiency is one of the key reasons the reaction can proceed at low concentrations.
- Enhanced Selectivity: The reaction is carefully tuned to avoid competition from other thiols and other reactive molecules in the bloodstream. Even in the presence of molecules like glutathione, the intended reaction between the prodrug components dominates.
- Structural Dynamics: The formation of the covalent dimer induces a conformational change that is favorable for binding to the STING receptor. This change is subtle yet significant, converting a benign pair of molecules into a highly active immune stimulant.
Impact on Immunotherapy: Lessons from Recent Preclinical Studies
The preclinical studies using zebrafish and mouse models not only underscore the technical feasibility of the two-component prodrug system but also offer important insights into how this technology can influence immunotherapy. Key observations include:
- Localized Immune Responses: In zebrafish xenografts, the addition of β-glucuronidase significantly improved the immune-stimulating effects, as measured by cytokine secretion and induction of apoptosis within the tumor.
- Improved Survival: Mouse tumor models have shown that when the active STING agonist is produced in situ, there is a marked improvement in tumor attenuation and survival rates. This response is comparable to, or even better than, the outcomes observed with conventional treatments.
- Pharmacokinetics and Tissue Distribution: Analysis using mass spectrometry has confirmed that, following treatment, the active compound remains confined primarily to the tumor. This minimizes adverse reactions in other organs, a key advantage over systemic administration of standalone STING agonists.
The Role of Advanced Imaging and Analytical Techniques
One of the most exciting developments in evaluating these targeted approaches is the availability of advanced imaging and analytical methods. Both confocal microscopy and mass spectrometry have played a key role in confirming that the active dimer is truly formed in situ. These techniques help to:
- Verify Localization: High-resolution imaging allows researchers to confirm that the reaction occurs where it is meant to, offering visual evidence of increased immune cell infiltration and tumor apoptosis.
- Monitor Kinetics: Time-course studies using LC–MS provide valuable information on the rate of dimer formation and its dissipation, thereby informing dose schedules and administration routes.
- Assess Safety Parameters: Thorough pharmacokinetic studies help ensure that the drug does not leak into undesired regions of the body, thereby reducing the risk of systemic adverse effects.
Integrating Patient-Centric Customizations into Prodrug Therapies
Even as this technology shows immense promise, the real-world application must account for individual variations in tumor biology. Patient-specific factors such as the level of enzyme expression and even the unique composition of their tumor microenvironment are super important when considering treatment plans.
Future clinical strategies might include:
- Biomarker Testing: Prior to treatment, patients could be tested for β-glucuronidase levels and other relevant parameters to ensure they are good candidates for prodrug activation.
- Personalized Dosing Regimens: Doses can be tailored based on the potential reaction rate between the prodrug components in each patient’s tumor, maximizing efficacy while minimizing side effects.
- Combination with Targeted Delivery Systems: Innovative methods such as nanoparticle carriers or microimplantable devices might be used to further refine and control the site and timing of prodrug activation.
Comparing Prodrug Strategies: A Look at the Alternatives
While the two-component prodrug approach represents a unique strategy, it exists alongside other methods aimed at generating drugs on-site within tumors. Below is a brief comparison of these strategies:
| Approach | Method of Activation | Strengths | Weaknesses |
|---|---|---|---|
| Two-Component Prodrug | Enzyme-triggered decaging + covalent dimer formation |
|
|
| Bioorthogonal Chemistry | Catalyst-mediated activation (e.g., palladium-catalyzed reactions) |
|
|
| Antibody–Drug Conjugates | Targeted delivery via antibody binding |
|
|
Overcoming the Overwhelming Landscape of Cancer Treatment
In the intricate world of oncology, treatment strategies must contend with many overwhelming challenges. When it comes to the two-component prodrug system, several important points stand out:
- Selective Activation Is Essential: By ensuring that the active compound is formed only at the tumor site, this method potentially minimizes many of the unintended effects seen in therapies where the drug circulates freely.
- It Addresses the Hidden Complexities of the TME: Tumors are known for their tangled issues and complicated pieces. A therapy that can specifically trigger its reaction in an environment rich with a particular enzyme offers a clear edge in addressing these subtle differences between diseased and healthy tissues.
- Opportunities for Future Enhancements: As researchers poke around the fine points of the chemistry and biological response, there exists ample room for further refinements that could make these therapies even more robust and widely applicable in various types of cancer.
Potential Risks and the Importance of Continued Research
No technology, no matter how innovative, comes without its own set of challenges. Even though early preclinical results are promising, certain nerve-racking risks need constant vigilance as we move toward clinical applications:
- Off-Target Activation: Although the design minimizes unintended activation, the variability in enzyme expression among patients means that even small levels of off-target activation could cause problems. Continuous monitoring and adjustment of dosage may be necessary.
- Immune Overstimulation: The immune system, while our powerful friend in fighting cancer, can sometimes go into overdrive. Careful balancing is needed to stir up a strong antitumor response while avoiding an inflammatory cascade that might harm the patient.
- Manufacturing and Scale-Up: The production of highly specialized molecules often involves a series of tricky parts and complex synthesis steps. Ensuring that these scientists can produce the compounds at scale without compromising their quality is essential for future clinical use.
Integrating Advanced Analytics for Safer Applications
Modern analytical techniques now offer researchers the tools needed to study these systems in great detail. For instance, high-resolution mass spectrometry and confocal imaging allow scientists to verify that the active components form only where they should. These methods also aid in:
- Real-Time Monitoring: Detailed imaging over time helps confirm that the tumor-specific activation is occurring correctly and that the active drug remains localized within the tumor.
- Quality Control: Sensitive assays enable rapid detection of any off-target activation, allowing for quick adjustments or dosing modifications before clinical side effects become problematic.
- Pharmacokinetic Profiling: Understanding how the drug behaves over time is key. Detailed concentration curves and tissue distribution assessments help ensure the therapy is both effective and safe.
Clinical Prospects and the Road Ahead
As discussions around precision medicine continue to evolve, tumor-specific prodrug strategies may soon find a place in the clinical toolkit. Their appeal lies in the clever manipulation of subtle details in both chemistry and biology to offer a treatment that is not only effective but also minimizes risks.
Looking ahead, several strategies could enhance the development of these therapies:
- Robust Preclinical Testing: Before widespread clinical adoption, extensive testing in animal models and potentially humanized systems will be essential to overcome the scary pitfalls associated with any new treatment modality.
- Enhancement of Delivery Platforms: Researchers are exploring novel nanoparticle carriers and guided delivery systems that could further refine how the components are administered. Such innovations can help make the treatment less invasive and more user friendly.
- Personalized Therapy Protocols: In the future, patients may receive a tailored combination of enzyme levels, precursor dosages, and administration schedules designed specifically for their tumor type. Advances in genomics and proteomics will be key here.
Key Takeaways: A Balanced View
After a closer look at the two-component prodrug system for tumor-specific STING pathway activation, a few key takeaways emerge:
- Innovative Mechanism: The strategy leverages enzymatic triggers and proximity-induced chemical reactions to produce a potent immune stimulant at precisely the right location.
- Potential for Enhanced Safety: By ensuring that the active drug is formed exclusively in the tumor, practitioners might be able to avoid many of the off-target effects that have limited the widespread use of STING agonists.
- Need for Personalization: Patient-to-patient variability in tumor enzyme expression means that bespoke therapies may be necessary for maximal benefit.
- Opportunities for Combination: This approach could complement existing therapies, thereby expanding the arsenal against resistant or advanced malignancies.
Taking the Wheel: Managing the Path from Bench to Bedside
The two-component prodrug system represents more than just an interesting chemist’s experiment—it is a tangible step toward refining cancer therapy. While the detailed steps of enzyme-triggered decaging and covalent dimer formation involve a number of tricky parts and tangled issues, the careful design and robust preclinical results give hope for the translation of these findings into clinical practice.
As scientists continue to sort out the fine shades between the active and inactive forms, and as engineers work to design practical administration routes for patients, the promise of this technology remains clear. With continued studies, stronger imaging techniques, and more advanced delivery systems, this strategy is poised to become a key element of future cancer therapies.
Final Thoughts: Optimism Amidst the Twists and Turns
While the journey from groundbreaking laboratory research to widely adopted therapy is often riddled with tension and unexpected hurdles, the tumor-specific two-component prodrug strategy stands out as a promising approach. Its ability to direct the potent action of STING agonists specifically to tumors, while minimizing systemic side effects, is critical in the space of precision oncology. The combination of chemical ingenuity, robust biology, and advanced analytics may well open new paths in cancer immunotherapy that were once considered too intimidating to navigate.
For clinicians, scientists, and patients alike, this approach signals a hopeful direction—a movement toward treatments that are not only effective in annihilating cancer but also gentle on the rest of the body. As the field continues to evolve, further research will undoubtedly refine these systems, help manage the complex bits of enzyme dynamics, and ultimately, lead to tailored, personalized therapies that can be safely and effectively implemented in diverse clinical settings.
Looking Forward: Embracing a New Era in Cancer Treatment
The future of cancer treatment is bright and full of opportunities. Innovations like the tumor-specific prodrug system remind us that the battle against cancer is being fought on multiple fronts—each addressing different, often subtle, aspects of tumor biology and treatment delivery. With careful attention to the fine points of enzyme activity, molecular reactivity, and immune modulation, researchers are well on their way to designing treatments that can genuinely transform patient outcomes.
As this technology matures, stakeholders will need to work together to translate laboratory findings into clinical realities. The collaboration of chemists, biologists, engineers, and clinicians will be key to overcoming the overwhelming challenges associated with scaling up production, optimizing delivery strategies, and personalizing treatment regimens. Together, these efforts are set to usher in a new era of targeted, safe, and effective cancer immunotherapy, inspiring hope for countless patients and reshaping the future of oncology.
In summary, the two-component prodrug strategy offers a fresh perspective on the role of immunostimulants in cancer treatment. By harnessing the power of localized enzyme activity and precise chemical reactions, this approach illuminates a path where the active drug can be synthesized exactly where it is needed—within the tumor microenvironment. While many of the underlying principles involve tricky parts and hidden complexities, the potential rewards are critical for patients facing the overwhelming challenge of cancer.
As we continue to take a closer look at the future of personalized cancer therapy, it is essential for all parties involved to recognize both the promise and the responsibility that comes with such innovations. Only through careful research, clinical validation, and compassionate patient care can these advances be transformed into real-world benefits, ensuring that the next generation of cancer treatments remains as safe as it is powerful.
Originally Post From https://www.nature.com/articles/s41557-025-01930-9
Read more about this topic at
Tumour-specific STING agonist synthesis via a two- …
Gene therapy for cancer using tumour-specific prodrug …