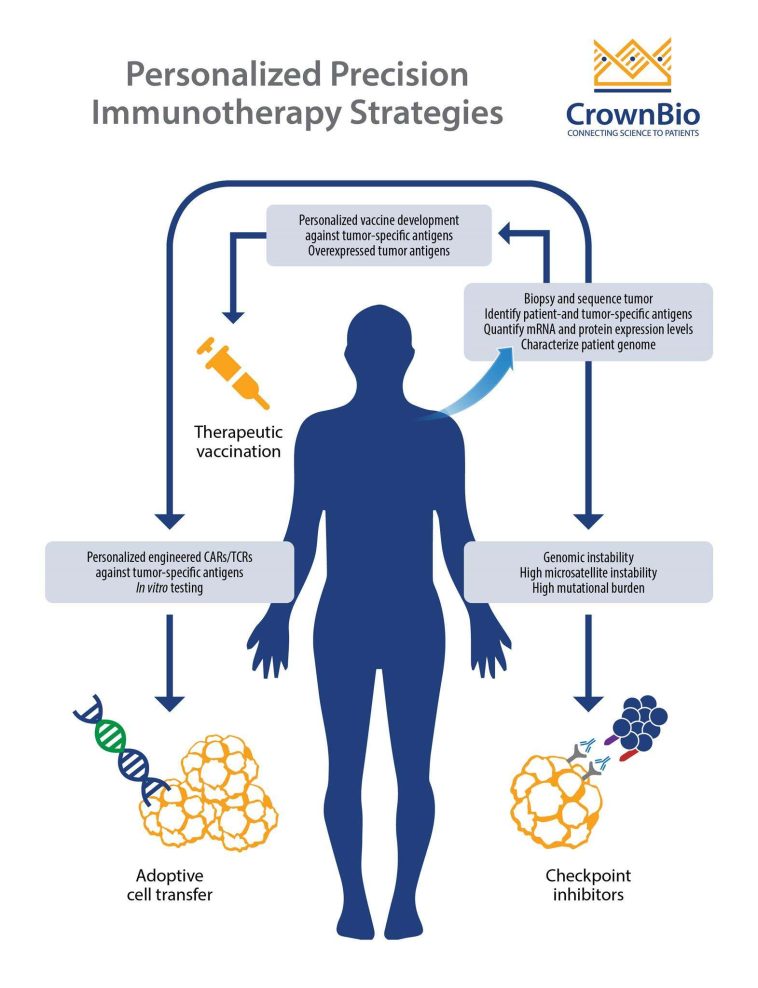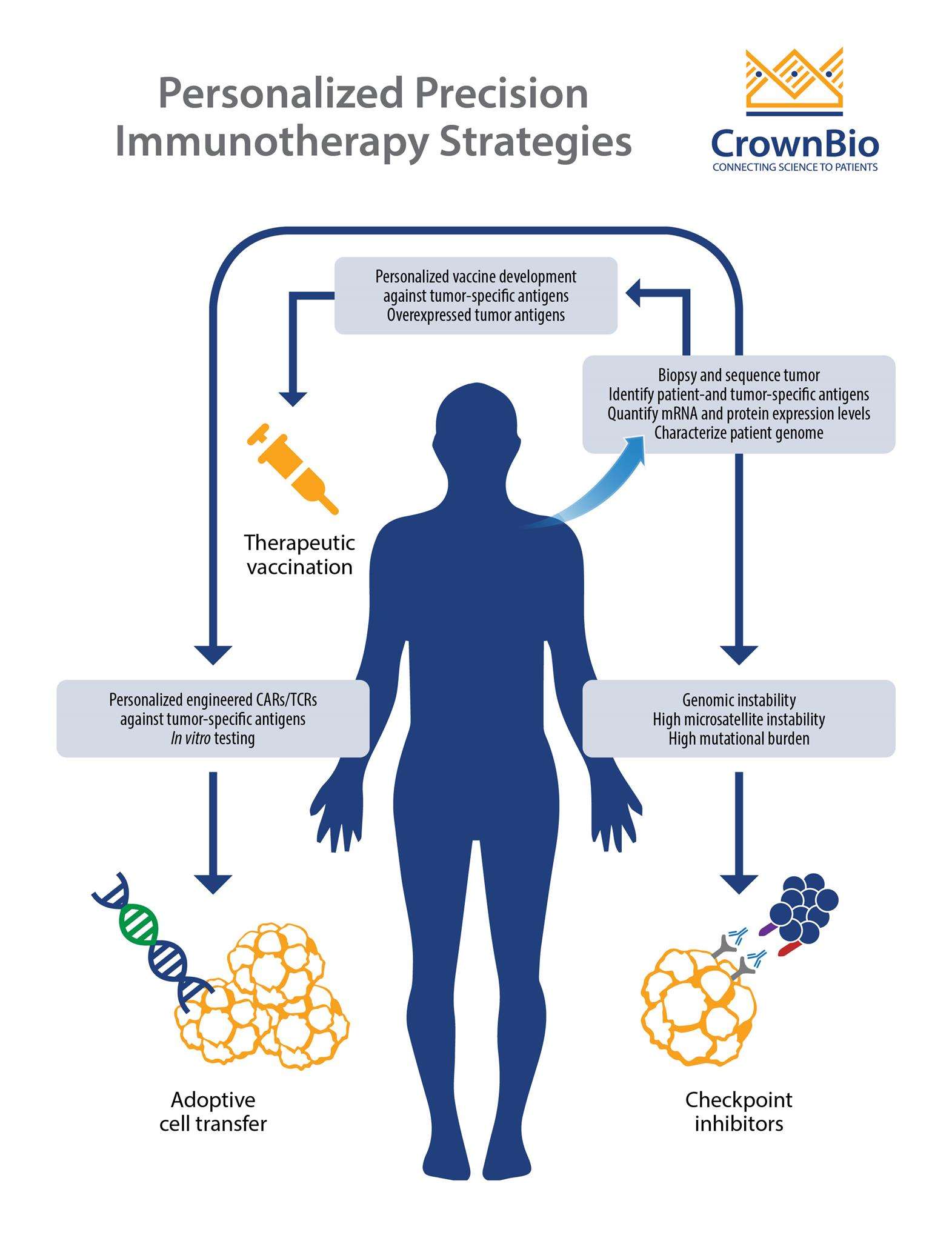
Introduction: The Changing Landscape of Cancer Treatment
The field of cancer treatment is experiencing a profound shift that is redefining traditional approaches. With an increasing emphasis on combining conventional therapies such as radiotherapy and chemotherapy with modern immunotherapies, oncologists and researchers are working through various tricky parts to improve patient outcomes. These combination strategies are not only aiming to directly attack tumors but also to harness the power of the immune system – essentially turning hostile cells into allies. In this editorial, we take a closer look at the current state and future promise of combinational cancer therapies, exploring their benefits, challenges, and the role of advanced digital planning tools.
With the advent of sophisticated research techniques and the rapid integration of artificial intelligence (AI) and multi-omics data into clinical practice, physicians can now figure a path through an increasingly tangled array of treatment options. While the journey toward fully personalized cancer care is full of problems and demanding twists and turns, the insights emerging from recent clinical studies provide both hope and a roadmap for future innovations.
Immunotherapy and Radiotherapy: A Dynamic Partnership
Radiotherapy Acting as an In Situ Vaccine
Conventional radiotherapy (RT) has long been a cornerstone of cancer treatment. However, recent evidence suggests that RT does more than just shrink tumors through direct cell damage. Studies have shown that localized irradiation can trigger immunogenic cell death (ICD), a process in which dying tumor cells release antigens and damage-associated molecular patterns (DAMPs). These elements, in turn, activate immune cells like dendritic cells, effectively turning the tumor into its own vaccine.
One key mechanism is the activation of the cGAS-STING pathway, which occurs when RT causes DNA damage. This pathway is crucial for the production of type I interferons, a group of proteins that play a significant role in mobilizing T-cell responses. By shifting the tumor immune microenvironment (TIME) from an immunosuppressive state to an immunologically active one, RT can stimulate both local and systemic anti-tumor responses. Still, there are some confusing bits: while RT can kick-start the immune system, it has also been known to upregulate checkpoint proteins such as PD-L1, promote regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs), and even trigger systemic lymphopenia.
Combining RT with Immune Checkpoint Inhibitors (ICIs)
The challenges posed by RT’s mixed effects have led researchers to look at combination therapies. In particular, the strategic use of immune checkpoint inhibitors (ICIs) alongside radiotherapy appears promising. By neutralizing the negative feedback loop induced by RT (for example, PD-L1 upregulation), ICIs can help maintain the boost from RT while simultaneously mitigating its suppressive qualities.
Studies in advanced non-small cell lung cancer (NSCLC) have shown that combining pembrolizumab—a well-known ICI—with RT not only improves progression-free survival (PFS) and overall survival (OS) compared to pembrolizumab alone but also leads to better distant tumor response rates. This research underscores the potential of harnessing RT’s immunomodulatory capacity when combined in a thoughtful, personalized manner.
Combination Therapies in Non-Small Cell Lung Cancer
Integrating Anti-Angiogenic Agents for Extended Progression-Free Survival
Non-small cell lung cancer (NSCLC) remains one of the areas where combinational therapeutics are showing pronounced benefits. A recent retrospective study investigated the addition of an anti-angiogenic agent called anlotinib to a regimen that already included immune checkpoint inhibitors and platinum-based chemotherapy. The study’s findings were noteworthy: patients who received this triple therapy exhibited a significantly longer median progression-free survival – by about 2.33 months – compared to those on a two-drug regimen.
This innovative approach indicates that integrating anti-angiogenic agents can play a key role in delaying disease progression. In this context, even the simpler combination of anlotinib with chemotherapy showed superior outcomes, suggesting that the anti-angiogenic component is critical, particularly for later-line NSCLC patients who have not responded adequately to standard treatments.
Benefits and Limitations in a Clinical Setting
To summarize and clarify the benefits and challenges of combination therapies in NSCLC, consider the following bullet list:
- Enhanced Efficacy: Combination treatments improve tumor response rates and overall survival.
- Tolerable Toxicity: While adding extra drugs increases side-effect risks, studies indicate that these can be managed effectively in a clinical setting.
- Need for Personalized Approaches: Not every patient benefits equally from combinational therapies, highlighting the necessity to identify specific biomarkers.
- Further Research Required: Additional randomized controlled trials are needed to confirm these retrospective findings and to determine optimal dosing and scheduling parameters.
Personalized Cancer Care: The Role of Biomarkers
Using PD-L1 Expression in Cervical Cancer Therapy
Another area witnessing notable advancements is recurrent or advanced cervical cancer. A meta-analysis has shown improved outcomes in progression-free survival (PFS) and overall survival (OS) when immune checkpoint inhibitors (ICIs) are used as part of the first-line therapy. A critical observation is that the effectiveness of ICIs in this setting is particularly evident in patients whose tumors have a higher expression of PD-L1 or who are diagnosed with squamous carcinoma histology.
This finding underscores the importance of considering PD-L1 status as an essential criterion in treatment selection. However, it is equally important to note that combining ICIs with conventional therapies may slightly increase the incidence of adverse events. Therefore, while PD-L1 is a key factor in refining treatment strategies, the decision-making process must also account for patient-specific tolerance and potential toxicities.
Baseline Lymphocyte Count as a Predictor in Hepatocellular Carcinoma
Biomarkers are equally important in liver cancer, specifically hepatocellular carcinoma (HCC). In a retrospective study involving HCC patients, baseline lymphocyte counts emerged as a promising marker. Patients with higher peripheral blood lymphocyte counts (PBLC) experienced better overall survival and progression-free survival when treated with a combination of tyrosine kinase inhibitors (TKIs) and PD-1 inhibitors.
This observation suggests that a simple blood test measuring lymphocyte levels could serve as a super important tool to identify patients most likely to benefit from targeted combination therapies. Using such an accessible measure, clinicians can better tailor treatment plans, improving outcomes while avoiding unnecessary toxicity and cost. Here is a concise table to illustrate the comparative outcomes based on PBLC levels:
| PBLC Level | Overall Survival (OS) | Progression-Free Survival (PFS) |
|---|---|---|
| High PBLC | Improved | Extended |
| Low PBLC | Lower | Shorter |
Adopting such predictive markers not only paves the way for individualized treatment but also helps minimize the risk of unnecessary side effects. It is a prime example of how personalized medicine continues to evolve within the realm of combinational cancer therapy.
Managing Side-Effects and Unpredictable Toxicities
Uncertain Outcomes: The Challenge of Immune-Related Adverse Events
While the promise of combinational treatments is immense, steering through the potential side effects remains a significant challenge. Immune checkpoint inhibitors, for instance, are known to trigger immune-related adverse events (irAEs). One striking example is the case of sintilimab-induced agranulocytosis, where a patient experienced dangerously low levels of neutrophils after treatment with an anti-PD-1 antibody. This condition, characterized by a severe drop in white blood cells, is a prime example of the unpredictable side effects that can arise.
Distinguishing the side effects of conventional chemotherapies from those directly linked to irAEs is not straightforward. To manage these nerve-racking toxicities, high doses of corticosteroids along with careful patient monitoring are necessary. The development and implementation of standardized protocols for predicting and managing these adverse events is one of the key challenges facing clinicians today.
Strategies for Safely Combining Treatments
Relevant strategies being deployed to manage and minimize unexpected side effects include:
- Enhanced Monitoring: Regular blood tests and imaging help detect early signs of adverse reactions.
- Risk Stratification: Techniques such as using baseline lymphocyte counts assist clinicians in identifying high-risk patients.
- Predictive Modeling: Incorporating AI-driven algorithms that analyze multi-omics data can simulate adverse outcomes before they happen.
- Tailored Management Protocols: Adjusting dosages and sequences based on patient response to treatment can mitigate risks.
These approaches collectively help manage the mixed signals from combination therapies and ensure that treatment improves a patient’s quality of life rather than compromising it due to overwhelming side effects.
Digital Treatment Planners: AI-Driven Individualized Cancer Care
Leveraging Artificial Intelligence and Multi-Omics Data
One of the most exciting prospects in modern oncology is the advent of digital treatment planners, a concept that uses artificial intelligence combined with clinical data from electronic health records and multi-omics profiling. This technology aims to create dynamic, individualized treatment strategies that consider every small detail about a patient’s tumor genomics, clinical parameters, and immune profile.
Imagine a scenario where an AI system takes into account all the little twists of each patient’s health profile and suggests a personalized combination of drugs, varying the sequence and doses in real time to maximize efficacy while preserving safety. This digital treatment planner could simulate various drug combinations and predict their potential outcomes, offering clinicians a digital roadmap to manage the increasingly tangled issues involved in cancer therapy.
Benefits and Future Potential of AI in Oncology
The key benefits of integrating AI into treatment planning include:
- Personalization: The system can adapt treatments based on individual response patterns and the nuanced details of tumor biology.
- Predictive Accuracy: By flagging patients at high risk for immune-related adverse events or other toxicities, the AI helps in risk mitigation.
- Dynamic Adaptability: As patient data accumulates, the treatment plan can be adjusted in real time, ensuring the regimen remains optimal throughout the course of therapy.
- Standardized Protocols: With continuous AI learning, hospitals and clinics can eventually work with standardized protocols that minimize variability in patient outcomes.
The integration of AI with digital treatment planners represents a leap forward—a transition from broad population-level evidence to finely tuned, individual-specific treatment approaches. This method is essential for managing the tricky parts of treatment selection and for reducing the nerve-wracking risk of severe side effects.
Challenges in Implementing Combination Therapies
Tackling the Tricky Parts of Treatment Selection
Despite the promise and power of combinational therapeutics, significant challenges remain that require further research and innovation. One of the most confusing bits involves the lack of robust, predictive biomarkers that can reliably indicate which patients will benefit from specific drug combinations. This gap in our understanding makes it tricky to decide on the right sequence and combination for each individual.
Moreover, the optimal dosing and scheduling of these combinations are still under investigation. While some studies suggest that neoadjuvant immunotherapy may offer enhanced efficacy, others highlight that the timing of the integration of chemotherapies, radiotherapies, and targeted agents is just as important as the drugs themselves.
Ensuring Equitable Access and Affordability
Another significant twist in the journey toward improved cancer care is the challenge of affordability and equitable access. The multidrug combination treatments that show the most promise are often expensive, leaving patients who lack proper insurance coverage or financial resources at a disadvantage. Researchers, clinicians, and policymakers must join forces to address these issues so that the benefits of combinational cancer therapies are available to all patients, not just a privileged few.
To break down this issue, consider the following table that outlines some of the key economic challenges and proposed measures:
| Challenge | Proposed Measure |
|---|---|
| High Cost of Multi-Drug Regimens | Implement drug price control and negotiate with pharmaceutical companies |
| Inequitable Access to Advanced Therapies | Government subsidies and patient assistance programs |
| Lack of Standardized Treatment Protocols | Collaborative efforts between research institutions and clinical experts |
Future Directions: The Road Ahead for Combinational Therapeutics
Moving from Sequential to Synergistic Treatment Strategies
The emerging trend in cancer therapy is moving away from the era of sequential, non-specific cytotoxic treatments and towards a synergistic approach that leverages a multi-target strategy on the tumor-immune ecosystem. By integrating therapies that are designed to complement each others’ actions, clinicians can create treatment regimens that not only effectively eliminate tumor cells but also enhance the patient’s immune response.
While the development of predictive models to identify the best patient subgroups is still a work in progress, the initial data are encouraging. For instance, the evidence that baseline lymphocyte counts and PD-L1 expression can serve as guides is a promising step towards more rational and personalized treatment choices.
Optimizing Treatment Sequences and Managing irAEs
Another key future direction is optimizing the sequence and timing of treatment components. The debate over whether to deliver these therapies concurrently or sequentially is ongoing. Some studies have shown that neoadjuvant immunotherapy, administered before the main treatment, can offer super important benefits by reducing tumor burden and priming the immune system. On the other hand, carefully timed sequential treatment can help mitigate overlapping toxicities and allow for recovery periods between drugs.
Equally important is the development of robust protocols for managing immune-related adverse events (irAEs). Future research is likely to focus on creating preventative strategies and using predictive models that can flag potential toxicities early in treatment. The aim is to ensure that the life-saving benefits of combinational therapies are not overshadowed by overwhelming side effects.
Collaboration Between Researchers, Clinicians, and Policy Makers
The path forward for combinational therapeutics is one that requires close collaboration across multiple fronts. Researchers and clinicians must work together to design and conduct rigorous clinical trials that further validate preliminary findings, while policymakers need to create environments where these advanced treatments can be widely and affordably accessed.
Some necessary steps include:
- Expanding Clinical Trials: More randomized controlled trials will help solidify the efficacy and safety profiles of combination therapies for a range of cancers.
- Standardizing Treatment Protocols: Developing consensus guidelines can minimize variability and improve clinical outcomes.
- Implementing AI-Based Decision Tools: Integration of digital treatment planners in clinical settings will allow for real-time adjustments and personalized strategies.
- Prioritizing Patient-Centric Solutions: Ensuring affordability and access to these advanced treatments is equally important for widespread clinical success.
Conclusion: A New Frontier in Cancer Therapy
In summary, the evolution of combinational cancer therapies marks a new chapter in the long fight against cancer. The synergy between traditional cytotoxic treatments and novel immunotherapies is redefining the standard of care through improved overall survival and progression-free survival across multiple cancer types. Researchers are steadily working through the tangled issues associated with biomarkers, treatment sequencing, and toxicity management to unlock the full potential of these multi-pronged strategies.
The role of radiotherapy in stimulating immunogenic cell death and activating immune pathways, particularly the cGAS-STING cascade, has provided a scientific foundation for these developments. Combined with the precision of immune checkpoint inhibitors and the promise of anti-angiogenic agents, this strategy offers a tailored approach that may transform several challenging cancers, including non-small cell lung cancer, cervical cancer, and hepatocellular carcinoma.
However, as we move deeper into this era of personalized medicine, significant challenges remain. The fine points of treatment selection require further exploration, and the unpredictable twists associated with immune-related adverse events demand innovative solutions. At the same time, the potential for digital treatment planners powered by AI gives hope that we can eventually create individualized, dynamic therapeutic regimens that account for every little detail of a patient’s condition.
Looking ahead, the integration of these emerging strategies into routine clinical practice will depend on collaborative efforts between the scientific community, healthcare providers, and policymakers. These stakeholders must sort out costing issues and ensure equitable access so that these promising treatments reach every patient who might benefit from them.
Ultimately, the new frontier of combinational cancer therapeutics is characterized by its potential to not only extend survival but also enhance the quality of life for cancer patients. With continued research, collaborative innovation, and a patient-centric approach, the transformation of cancer into a more manageable, chronic condition may soon become a reality.
This journey is undoubtedly full of tricky parts and tangled issues, but through tactical approaches, careful monitoring, and the aid of next-generation digital planning, the future of cancer treatment looks exceptionally promising. It is an exciting time to be part of a field that is increasingly capable of blending the best of traditional and modern therapies into a coherent and life-saving strategy.
Originally Post From https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2025.1729774/abstract
Read more about this topic at
Combinatorial Approach to Improve Cancer Immunotherapy
Synergistic cancer immunotherapy combines MVA-CD40L …