Revitalizing T Cells: A New Era in Cancer Immunotherapy
In recent years, the fight against cancer has witnessed revolutionary advances as scientists strive to harness the body’s own immune system. The latest breakthrough in reawakening exhausted T cells has the potential to supercharge cancer immunity by addressing the tricky parts of immune cell fatigue. As we take a closer look at this transformative discovery, we uncover how interrupting tumor signals can keep T cells active and offer promising hope for patients who have not seen an adequate response to conventional immunotherapies.
For decades, oncologists and immunologists have wrestled with the complicated pieces of cancer treatment. Despite remarkable progress with checkpoint inhibitors, many patients face the overwhelming challenge of T cell exhaustion—a state where immune cells, though present and primed, become tired after continuous exposure to tumor signals. This opinion editorial examines recent research from Weill Cornell Medicine that demonstrates how disrupting a particular molecular cue can help maintain T cell activity, potentially re-energizing the body’s natural defense against tumors.
Understanding T Cell Exhaustion in Cancer
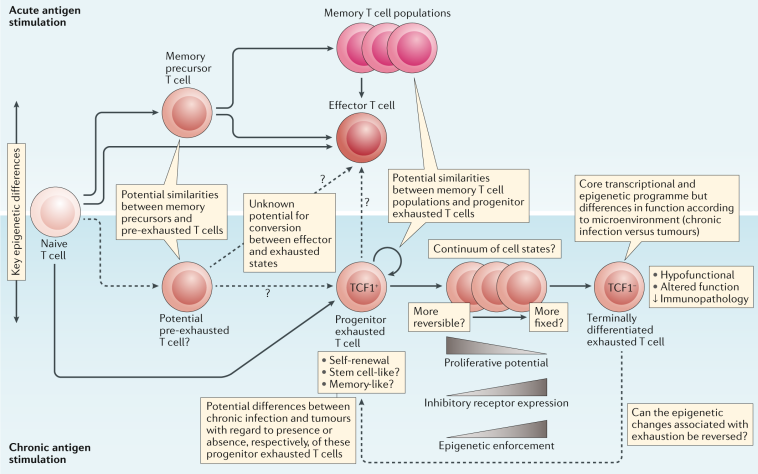
T cell exhaustion occurs when the immune system is consistently engaged in battling long-term infections or persistent tumor growth. In these scenarios, although T cells can recognize harmful cells, they lose their effectiveness over time due to a series of confusing bits in the immune response. As exhausted T cells no longer attack tumors with full force, the body’s ability to combat cancer diminishes.
This new research dives in to expose how tumors exploit a specific molecular signal to weaken T cells. Recognizing that these signals are critical in suppressing immune function, the study focuses on the role played by molecules such as CD47 and thrombospondin-1. By digging into the scientific evidence, researchers have established that when these signals are blocked, T cells can regain their original, potent capacity to fight cancer.
Immune Checkpoints and Their Role in Cancer Therapy
Over the past decade, immune checkpoint inhibitors have emerged as a crucial development in oncology. These therapies work by removing the brakes on the immune system, thereby enhancing the attack on cancer cells. However, not all patients experience lasting benefits. The diminishing effects seen in some treatments are partly due to the high levels of exhaustion observed in T cells.
Recent studies have drawn attention to the fact that while surface proteins such as PD1 were once considered the primary culprits in T cell exhaustion, newer research points to the involvement of CD47. This discovery adds another layer of complexity to our understanding of the tumor microenvironment, as it reveals that cancer cells use multiple pathways to evade immune detection. By purposely blocking these pathways, clinicians hope to revive T cell activity and improve outcomes in cancer therapies.
How CD47 and Thrombospondin-1 Influence T Cell Function
One of the most striking observations from the recent study is the dual role played by the CD47 molecule. Not only do cancer cells exploit CD47 as a “don’t eat me” signal to shield themselves from immune attack, but activated T cells also express CD47. Interestingly, when T cells become exhausted, this molecule is upregulated to very high levels, further contributing to the loss of immune efficacy.
Research has demonstrated that when T cells are deprived of CD47, they show superior tumor-fighting abilities, particularly in melanoma and colorectal cancer models. This points to the fact that the interaction between CD47 and its binding partner, thrombospondin-1, is more than a simple “on/off” switch. Instead, it represents a nuanced system with subtle details that affect T cell performance over the long haul.
To illustrate, consider the following table, which outlines the two main components involved in this immune suppression mechanism:
| Component | Function in Immune Response | Impact When Overexpressed/Activated |
|---|---|---|
| CD47 | Acts as a signal on immune and cancer cells | High expression can lead to T cell exhaustion and immune evasion |
| Thrombospondin-1 | Binds to CD47 to regulate cell interactions | Increases immune suppression when elevated in the tumor microenvironment |
This table helps clarify how the high levels of CD47 and thrombospondin-1 work together to create confusing bits in the immune system’s ability to fight cancer. The research shows that disrupting their interaction could lead to significantly improved T cell function, offering a new pathway to control cancer.
Optimizing T Cell Response in Melanoma Treatment
Melanoma, an aggressive form of skin cancer, has often been at the forefront of adopting immune checkpoint inhibitors. However, even in melanoma, the nerve-racking challenge of T cell exhaustion remains a glaring obstacle. By utilizing a peptide known as TAX2, scientists managed to interrupt the troublesome connection between thrombospondin-1 and CD47. This intervention not only maintained T cell activity but also significantly slowed tumor growth in preclinical models.
The use of TAX2 is especially promising as it acts as a proof-of-concept for blocking detrimental immune signals. Early experiments showed that T cells in animals treated with TAX2 remained more active and produced greater amounts of immune-boosting cytokines. Furthermore, these enhanced T cells were better at infiltrating tumor sites, a factor that is critically important in mounting an effective immune response.
Key takeaways in optimizing T cell response include:
- Maintaining T cell activity: Blocking specific inflammatory signals can rejuvenate T cell function.
- Enhancing cytokine release: Increased cytokine production can improve the overall immune response.
- Improved tumor infiltration: Active T cells that can reach and penetrate tumor sites are crucial for sustained cancer immunity.
These findings pave the way for new treatment strategies in melanoma, especially for patients who have become resistant to current immunotherapy protocols.
Understanding Immune Checkpoint Blockade in Colorectal Cancer
Colorectal cancer is another area where immune-based therapies have shown promise. However, like in melanoma, the efficacy of these treatments is often hindered by the high levels of exhaustion observed in T cells. Researchers have found that when the CD47-related pathway is disrupted, T cells regain essential, or super important, capabilities to combat tumors.
In colorectal cancer models, combining treatments that block both PD1 and CD47 pathways resulted in T cells that were markedly more effective in destroying cancer cells. This dual approach suggests that a multi-targeted therapy might be the key to overcoming the persistent suppression mechanisms employed by tumors.
The following bullet list summarizes the potential benefits of this combined therapeutic strategy:
- Enhanced T cell rejuvenation through blocking dual inhibitory signals.
- Increased persistence of active T cells in the therapeutic window.
- Potential for overcoming resistance to single-agent checkpoint inhibitors.
- Reduced tumor growth rates in preclinical studies.
The combination of these strategies creates a powerful arsenal against cancer, allowing oncologists to figure a path to more effective treatments for colorectal cancer patients.
Dealing with the Tricky Parts of Tumor Immune Evasion
Tumors are notoriously clever in evading the body’s immune defenses. They use a series of tangled issues—ranging from the expression of inhibitory proteins to the manipulation of immune signaling pathways—to create an environment where T cells are forced into a state of exhaustion. Understanding these twists and turns is essential for developing strategies that can reverse immune suppression.
In the context of the recent study, tumors were found to manipulate the immune system using the molecule thrombospondin-1 in association with CD47. This duo creates a nerve-racking scenario where T cells lose their ability to effectively engage with cancer cells. The study’s approach to interrupt this problematic interplay with the TAX2 peptide opens up new avenues for reducing tumor immune evasion.
To better illustrate the challenge, consider the following key points:
- Persistent tumor signals: Continuous exposure to tumor-derived signals gradually wears out T cells.
- Adaptive changes in immune cells: T cells, when activated, upregulate proteins like CD47, which later may contribute to their exhaustion.
- Dual pathways of suppression: Both PD1 and CD47 play roles in dampening the immune response, meaning that targeting one pathway might not be enough to fully restore T cell function.
Such challenges highlight the pressing need to sort out the fine points of immune regulation in cancer. By understanding and then carefully interrupting these immune suppressive mechanisms, researchers hope to develop more robust immunotherapies that are less susceptible to resistance.
Diving Into the Future: New Horizons in Cancer Immunotherapy
The innovative approach of using the TAX2 peptide to block the connection between thrombospondin-1 and CD47 marks a significant milestone. It serves as a window into future cancer treatments, where the focus will be on reviving the body’s own defenders rather than solely relying on external interventions. With further research, similar strategies could be tailored individually to enhance patient outcomes across a variety of tumor types.
Looking ahead, the research community is eager to pinpoint additional upstream and downstream modulators that play into this problematic pathway. The goal is to develop techniques that are both selective and safe, allowing clinicians to harness reactivated T cells to their fullest potential without triggering undesirable side effects.
Future clinical trials may see the combination of checkpoint inhibitors with therapies targeting both PD1 and CD47 pathways. In doing so, the hope is to achieve a more durable immune response that is capable of keeping the tumor at bay for longer periods. This could be especially transformative for patients whose cancers have developed resistance to conventional therapies.
Key areas to watch in future research include:
- Identification of new molecular targets: Expanding beyond just PD1 and CD47 to include other proteins involved in immune suppression.
- Development of combination therapies: Using agents that simultaneously attack multiple pathways involved in T cell exhaustion.
- Personalizing treatment: Adjusting therapeutic approaches based on the unique immune profiles of individual patients.
- Improved monitoring techniques: Advancing diagnostic tools to better assess T cell function and exhaustion levels in real-time.
Exploring these directions will likely reduce the overwhelming challenges posed by the tumor microenvironment and open up new, more effective treatment options.
Overcoming the Overwhelming Challenges in Cancer Treatment
While the new findings bring great promise, it is important to acknowledge the nerve-racking reality that challenges remain. Tumors are masterful at finding ways to outsmart the immune system, and no single therapy is likely to be a magic bullet. The path ahead is laden with subtle parts and tangled issues that must be understood and addressed through careful scientific inquiry.
It is essential that medical researchers and clinicians continue to get into every detail of these immune suppression pathways. By combining multiple approaches and leveraging the emerging potential of peptides like TAX2, the future of cancer treatment looks optimistic despite the current full of problems state of affairs. Studies like this one serve as a reminder of the need for persistence and innovation in oncology.
The road to fully effective cancer immunotherapy is on edge with tension, but each breakthrough adds another building block toward creating more resilient treatment protocols. The new research suggests that by focusing on reawakening T cell activity and contending with the battling twists and turns of immune evasion, the journey to defeat cancer is progressing steadily.
Integrating Immunotherapy into Everyday Medical Practice
As promising as the new strategies may be, integrating them into everyday medical practice will require a concerted effort from researchers, clinicians, and regulatory bodies alike. The transition from laboratory research to clinical application always involves navigating a maze of scientific, logistical, and regulatory hurdles.
To facilitate this integration, multidisciplinary collaboration is essential. Oncologists, immunologists, pharmacologists, and even bioengineers must work together to develop, refine, and test new therapies that aim to keep T cells active. This collaboration is not only key to unlocking the potential of immune-based therapies but also to sorting out the practical challenges that emerge along the way.
For example, successful clinical trials of combination therapies will require:
- Robust patient recruitment strategies to ensure diverse representation.
- Comprehensive monitoring protocols to track T cell responses and side effects.
- Adaptive trial designs that can pivot based on early results.
- Collaborative funding models that bring together public, private, and philanthropic resources.
By working through these challenges, we can create a future where the immune system itself is equipped to stand up to cancer, potentially reducing the need for more invasive or toxic treatments.
Personal Reflections on the Promise and Pitfalls
As an observer in the field of healthcare and immunotherapy, I find the emerging research both exhilarating and cautiously optimistic. The ability to reinvigorate T cells by merely blocking a signal that tumors exploit is revolutionary. Yet, like all groundbreaking discoveries, there are inherent risks and pitfalls that we must address as we build on this knowledge.
For instance, while the TAX2 peptide offers a clear proof-of-concept, the long-term effects of interfering with the thrombospondin-1 and CD47 connection need thorough investigation. It is critical to ensure that discontinuing this pathway does not inadvertently promote other harmful responses, such as uncontrolled inflammation. The balance between reactivating the immune system and preventing adverse side effects will require careful dose management and patient monitoring.
Moreover, as we integrate these findings into practical treatments, communication and education will be key. Patients and healthcare providers alike need clear and accessible information about how these therapies work and what the potential outcomes might be. Transparency in the development process can help to build public trust and ensure that innovative treatments are widely accepted and used effectively.
In summary, while the journey to fully effective immunotherapy is on edge with mixed challenges, the renewed focus on reawakening T cells has the potential to bring about a major shift in cancer treatment paradigms. This research is a call to action for scientists and clinicians to dig into the fine points of immune signaling and develop younger, more robust approaches to cancer therapy.
Concluding Thoughts: A Step Forward in the Battle Against Cancer
In conclusion, the latest research into reawakening exhausted T cells represents a significant leap forward in understanding how to combat cancer’s tricky defense mechanisms. By focusing on the interplay between CD47 and thrombospondin-1, scientists have opened the door for a new class of immunotherapies designed to keep T cells active in the tumor microenvironment.
This breakthrough underscores the importance of combining innovative scientific approaches with practical clinical applications. As our understanding deepens, future treatments may well hinge on the ability to preserve and enhance T cell function—ultimately transforming cancer from a formidable foe into a manageable condition.
Moving ahead, it is clear that the success of these new strategies will depend on collaboration, careful study of the subtle details of immune regulation, and the relentless pursuit of answers to the tangled issues of tumor immune evasion. With optimism and caution in equal measure, the scientific community is steering through a period of extraordinary promise.
For patients, clinicians, and researchers alike, the prospect of revitalized T cells represents a beacon of hope in an area full of problems and unexpected challenges. The journey to fully harness the body’s immune power is not without its nerve-racking moments, but each step forward brings us closer to a future where the immune system itself can be the ultimate weapon against cancer.
In this brave new world of cancer immunotherapy, staying informed and adaptable is key. As research continues to expose the hidden complexities of immune suppression, the path to creating more effective, patient-friendly treatments becomes clearer. In the end, it is the convergence of innovative science, clinical expertise, and patient advocacy that will light the way to enduring progress in our battle against cancer.
With so much at stake, the ongoing dialogue between laboratory research and clinical practice will be the driving force behind breakthroughs that can save countless lives. Today’s discoveries, like the reactivation of exhausted T cells, provide a glimpse into tomorrow’s potential—a future where fighting cancer is not just about managing a disease, but truly liberating the power of the human immune system.
Looking Ahead: The Road to Expanded Immunotherapy Applications
Looking forward, the integration of innovative therapies into standard practice remains a critical priority. The recent study not only emphasizes the importance of targeting specific molecular pathways but also illustrates the benefit of adopting combination therapies to tackle cancer from multiple angles. This is a crucial insight for the future of personalized medicine, where every patient’s treatment can be tailored to their unique immune profile.
As emerging treatments are tested in clinical settings, the medical community must maintain a focus on safety, efficacy, and accessibility. The use of combination approaches, such as pairing PD1 inhibitors with agents that disrupt CD47 signaling, could provide a broader therapeutic window for patients who face aggressive or resistant forms of cancer. This multi-pronged strategy might prove essential in ensuring that immunotherapies are both effective and sustainable over time.
Furthermore, future research should continue to explore:
- Biomarker development: Finding reliable indicators for T cell exhaustion and activation to better guide treatment decisions.
- Patient stratification: Classifying patients based on their immune profiles to determine who may benefit most from combination therapies.
- Long-term outcomes: Investigating the durability of T cell responses following treatment interruption of suppressive pathways.
- Side-effect management: Developing strategies to mitigate any unintended consequences from long-term pathway blockade.
These research avenues are essential for paving the way toward treatments that not only combat cancer effectively but also improve overall quality of life for patients. The integration of advanced immunotherapy tactics into routine cancer care promises to transform the prognosis for many individuals previously facing a bleak outlook.
Ultimately, the ultimate aim remains simple yet profoundly challenging: to make immune-based therapies available to every patient, regardless of cancer type or stage. By continuing to explore and address the hidden complexities and tricky parts of immune regulation, the medical community inches closer to this goal every day.
Final Reflections on the Evolution of Cancer Immunotherapy
The journey to understanding and overcoming T cell exhaustion is emblematic of the broader struggle against cancer. Each step forward—each new discovery—sheds more light on the subtle details that govern the immune system’s interactions with malignancies. Although the road is filled with tangled issues and nerve-racking challenges, the collective efforts of researchers and clinicians continue to inspire hope.
Reflecting on the progress made so far, it is evident that reawakening exhausted T cells is not just a scientific breakthrough; it represents a new chapter in the history of cancer treatment. Through a combination of innovative research, collaborative effort, and the persistent drive to overcome obstacles, we are witnessing the evolution of immunotherapy into a cornerstone of modern oncology.
In closing, let this new approach to reactivating T cells remind us that even in the face of overwhelming challenges, there is always a way to find your path forward. As we work through the little details and the fine shades of immune regulation, every discovery is a stepping stone toward more effective, personalized, and life-saving treatments. The future of cancer care is bright, and the promise of revitalizing the immune system offers hope to patients and clinicians across the globe.
With continued research and a steadfast commitment to innovation, the dream of harnessing the full potential of the immune system is closer than ever. The reawakening of exhausted T cells is just the beginning—an inspiring milestone in our collective journey toward a world where cancer can be controlled, managed, and ultimately defeated.
Originally Post From https://www.sciencedaily.com/releases/2025/11/251120002828.htm
Read more about this topic at
Reviving Exhausted Immune Cells Boosts Tumor Elimination
Reviving Exhausted T Cells Sparks Powerful Cancer …