Personalized mRNA Vaccines: The Future of Cancer Treatment?
Advancements in medical science continually push the boundaries of what treatments can achieve. One of the most promising developments of recent years is the use of personalized mRNA vaccines to treat cancer. With cancer remaining one of the leading causes of death despite significant treatment advancements, these tailor-made vaccines offer a uniquely targeted approach. In this opinion editorial, we look into how personalized mRNA vaccines work, the process behind their development, and the challenges – including funding issues and public skepticism – that could shape their future.
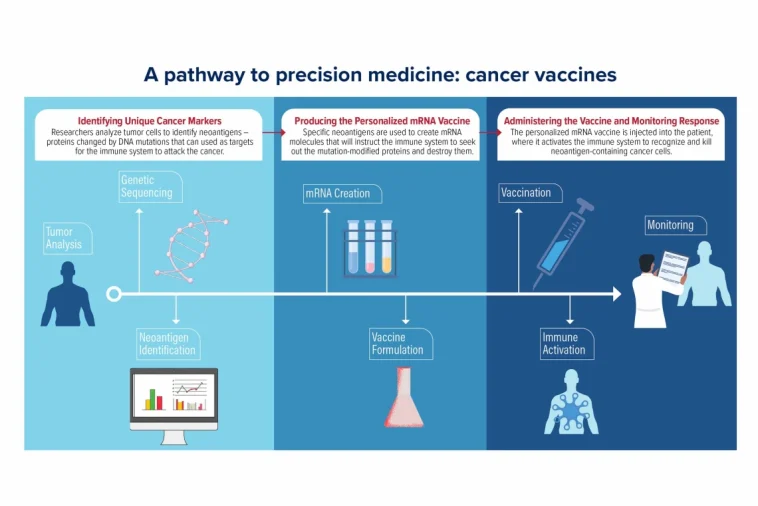
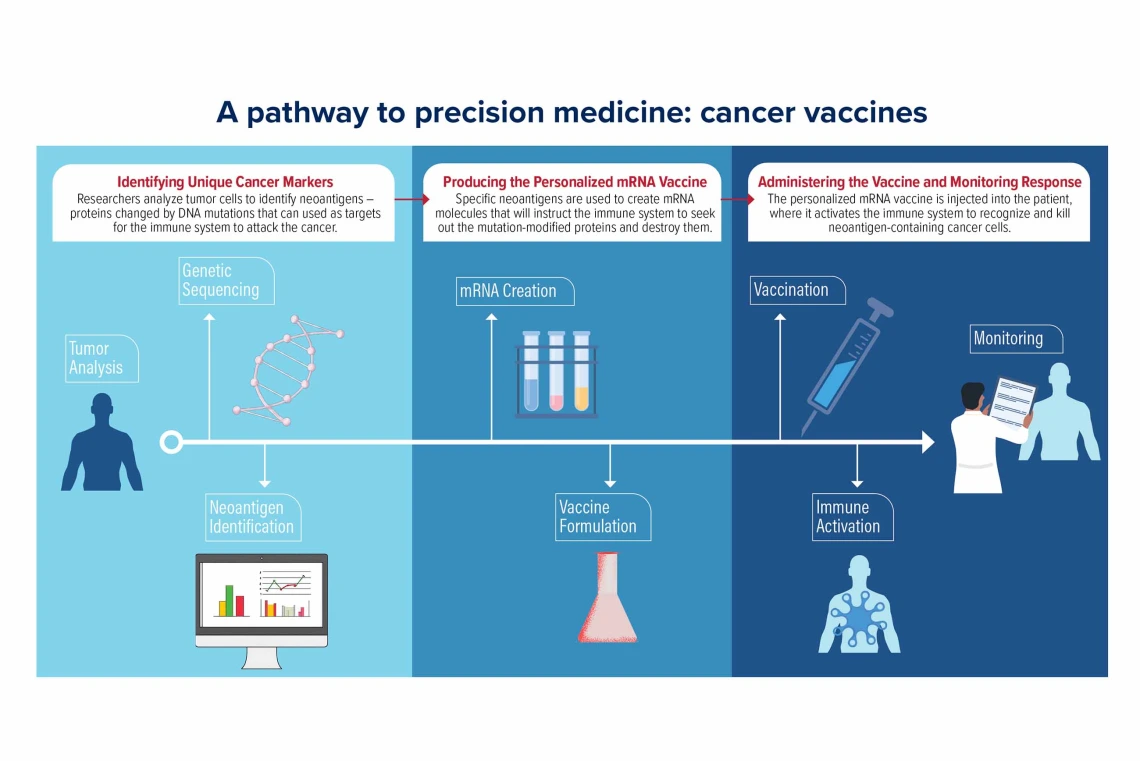
Personalized mRNA vaccines are designed to instruct the immune system to recognize and fight cancer cells based on the unique mutations found in a patient’s tumor. The process, although it seems like it comes straight from a science-fiction narrative, is grounded in decades of research. By harnessing the body’s own mechanisms to identify abnormal proteins, these vaccines provide a way for the immune system to learn exactly which cells to target.
Understanding the Process: How Tailored mRNA Vaccines are Manufactured
When a patient’s tumor is surgically removed, a small portion of the tumor tissue is carefully collected and sent on a detailed journey through the laboratory. Every step – from quick freezing in ultra-cold conditions to fine slicing and staining – ensures that the integrity of the genetic material remains intact. These samples are later sequenced to pinpoint the mutations that differentiate the cancer cells from healthy tissue.
This sequencing gives researchers the data they need to create a custom mRNA vaccine. In simple terms, the mRNA in the vaccine acts like an instruction manual for the immune system. It teaches immune cells to detect the mutant proteins, or “neoantigens,” that are unique to the tumor. Once the immune system learns to recognize these mutant proteins, it is ready to eliminate any cells bearing them.
Steps in the Vaccine Development Process
- Tissue Processing: After the tumor is removed, a small piece of it is immediately processed to preserve the genetic material.
- Genetic Sequencing: Scientists analyze the tissue for specific mutations that produce abnormal proteins.
- Neoantigen Identification: Not every mutation is suitable. Researchers have to pick out the mutations that lead to neoantigens most likely to trigger an immune response.
- Designing the mRNA: The selected genetic sequence is then formatted into mRNA, which is packaged into tiny fat particles for delivery.
- Administering the Vaccine: Once ready, the personalized vaccine is given to the patient via injection, aimed at priming the immune system.
The process is not without its tricky parts and tangled issues. For instance, the sequencing of a tumor tissue is just a snapshot in time; cancer can evolve and change, meaning that the target of the vaccine might not remain the same over time. Furthermore, each patient’s cancer is unique – a constant reminder of the twists and turns in personalized medicine.
Integrating Modern Science with Personalized Treatment Options
The idea of personalized vaccines represents a convergence of several modern advancements: genetic sequencing, artificial intelligence, and innovative vaccine delivery systems. Years of research on mRNA technology, which initially focused on infectious diseases such as COVID-19, have now been adapted to tackle cancer. Through the use of computational tools, scientists have been able to dig into the fine points of a tumor’s mutations and work through the complicated pieces that make each case unique.
This method capitalizes on the natural ability of our immune system to recognize foreign invaders. By turning the immune system against the cancer itself, personalized mRNA vaccines offer an approach that is both targeted and potentially far less toxic than traditional treatments like chemotherapy and radiation, which often hit healthy cells along with cancer cells.
Benefits Over Traditional Cancer Therapies
- Targeted Approach: Unlike chemotherapy or radiation, which affect both healthy and cancer cells, personalized vaccines help guide the immune system directly to the problem, reducing collateral damage.
- Reduced Side Effects: With traditional cancer treatments, patients often experience overwhelming side effects. Personalized mRNA vaccines aim to minimize these issues.
- Adaptability: The flexibility of mRNA technology means that once a platform is established, it can be quickly adjusted to target different kinds of cancer.
- Long-Lasting Immune Response: Early trials have shown that a robust immune response can be maintained over an extended period, suggesting long-term benefits.
Efforts and Challenges in Clinical Trials
Early clinical trials have been critical in showcasing the promise of personalized mRNA vaccines. For instance, in a small phase 1 clinical trial, patients with pancreatic and melanoma cancers received vaccines specifically tailored to their tumors. Many participants demonstrated a significant immune response, and a number of patients remained cancer-free years after the treatment.
However, these trials are not without hurdles. The scientific community continues to face many confusing bits when it comes to determining the precise dosage, timing, and administration protocols for these vaccines. The process is as nerve-racking as it is groundbreaking: ensuring that the immune system recognizes the needed targets without triggering unintended consequences requires painstaking fine-tuning.
Key Considerations in Clinical Research
- Patient Selection: Identifying those most likely to benefit from a personalized approach remains a challenge.
- Dosage Optimization: Studies strive to determine the best dosage levels that will elicit a strong immune response while minimizing side effects.
- Combination Therapies: Personalized vaccines are often administered alongside standard treatments. Researchers continue to investigate how best to combine these therapies to maximize outcomes.
- Long-Term Monitoring: Ongoing observation is critical to understanding how the vaccines perform over several years, particularly when considering tumor evolution.
For many researchers, the results so far are encouraging, but the scientific community remains cautious. They know that while the early data is promising, there is a long road ahead. Many of the underlying subtle parts of the tumor-immune system interactions are still being figured out. This further underlines the importance of continuing to support extensive and long-term clinical trials.
Funding Pressures and Regulatory Hurdles
The journey from the research lab to the patient’s bedside is not solely a scientific challenge – it is also chock-full of financial and political twists and turns. In recent years, reductions in federal funding for cancer research and increasing regulatory pressures have threatened to slow the progress of innovative treatments like personalized mRNA vaccines.
For decades, much of the pioneering work in cancer research has been funded by government agencies such as the National Institutes of Health (NIH). However, changes in funding policies and shifting political priorities have led to budget cuts. One striking development has been the move to limit indirect research contributions, meaning that fewer resources are available to cover the many overhead costs associated with high-caliber research facilities and equipment.
The Impact of Funding Cuts on Research
| Aspect | Traditional Funding Scenario | Current Challenge |
|---|---|---|
| Indirect Funding | 50-60% extra funds for overhead | Limited to 15%, reducing resources |
| Research Capacity | Ability to support robust, long-term studies | Reduced hiring and slower progress |
| Clinical Trials | Well-funded multi-year trials | Need to cut back and shorten trial periods |
These financial challenges have real-world implications. With fewer resources available, research institutions are forced to scale down experiments, reduce postdoctoral hires, and in some cases, even cut back on the size of their incoming medical classes. For the field of personalized mRNA vaccines, which requires close collaboration between scientists, clinicians, and biotechnologists, these funding cuts add an additional layer of tension that can slow progress.
Moreover, regulatory hurdles have also come into play. In certain political climates, skepticism surrounding vaccine technologies has led to increased scrutiny of mRNA research. Legislative actions and shifts in regulatory agency leadership have at times cast doubts on the future of vaccine research, even as the global community reaps the benefits of COVID-19 vaccine developments.
Public Perception and the Role of Vaccine Hesitancy
The development of personalized mRNA vaccines is not occurring in an information vacuum. Public perception of vaccines, shaped in part by decades of debate and misinformation, plays a crucial role in how these new therapies are received. While the COVID-19 pandemic accelerated public familiarity with mRNA technology, it also heightened vaccine hesitancy among certain segments of the population.
Critics and skeptics of vaccine safety have at times spread concerns about mRNA technologies, even though the evidence overwhelmingly supports their safety and efficacy. The skepticism not only affects public willingness to receive vaccines but also influences governmental and regulatory decisions on funding and support for research. The situation is a tangled mix of scientific innovation colliding with political sentiment.
Strategies to Overcome Public Hesitancy
- Enhanced Communication: Scientists and public health officials need to make information accessible and relatable. Using everyday language can help demystify complex scientific developments.
- Community Engagement: Involving patient advocacy groups and community leaders adds a layer of trust that is crucial in shifting opinions.
- Transparency and Education: Detailed explanations of the small distinctions between traditional vaccines and novel mRNA-based approaches can reassure the public that every measure is taken to ensure safety.
- Highlighting Success Stories: Personal testimonies from trial participants and stories of cancer survivors can put a human face on what might otherwise be seen as overly technical science.
In regions where skepticism is particularly strong, managing your way through public sentiment is almost as vital as managing the science itself. By establishing clear, open channels of communication and providing evidence-based responses, proponents of the technology hope to smooth over the nerve-wracking concerns that linger in the public mind.
The Role of Artificial Intelligence in Accelerating Progress
One of the most promising elements of modern mRNA vaccine development is the integration of artificial intelligence (AI) in both research and manufacturing processes. AI systems are now being used to parse through the mountain of data generated by tumor sequencing and to figure a path toward identifying the most promising neoantigens for targeting.
These machines can analyze massive datasets quickly, identifying subtle details and fine shades within the genetic code that might be missed by human researchers. Computational tools help to dig into the little details, making sure that the vaccine targets are both distinct and effective. This level of analysis is critical to ensure that the personalized vaccine is tailored not only to the individual’s current state of disease but also potentially adaptable to future changes in the tumor’s profile.
Advantages of AI in Vaccine Manufacturing
- Speed: AI drastically cuts down the time required to analyze tumor data.
- Precision: Algorithms can spot the subtle signs that distinguish one mutation from another, leading to more refined vaccine designs.
- Consistency: Automated quality-control processes ensure that even the tiniest batches of personalized vaccines meet strict regulatory standards.
- Cost-Effectiveness: Streamlined production processes reduce overall manufacturing costs, potentially making these personalized treatments more widely accessible.
Efforts at companies like Moderna and BioNTech illustrate how AI is already beginning to reengineer production lines established during the COVID-19 pandemic. By adopting innovative robotics and streamlined workflows, these companies are setting the stage for a revolution in personalized medicine. Their work not only underscores the inherent promise of mRNA-based cancer vaccines but also highlights the potential for new manufacturing paradigms that could benefit various areas of healthcare.
Societal Implications: The Promise and the Risks
The move towards personalized mRNA vaccines carries significant societal implications. On one hand, the ability to provide custom-tailored treatment for cancer could mean dramatically improved outcomes for patients who have historically had few options. On the other, the political and funding challenges, compounded by widespread vaccine hesitancy, pose real threats to the continued development and adoption of these therapies.
For many patients, personalized vaccine therapies could mean the difference between a life shadowed by recurring cancer and one enriched by the spontaneity of renewed health and hope. For instance, consider the story of a patient in an early clinical trial who managed to maintain an exceptionally strong immune response for several years after the treatment. This single testimonial, while anecdotal, captures the potential of the technology to redefine cancer care.
Yet it’s equally important to acknowledge that the progress of these therapies is closely tied to public policy and societal support. Political decisions regarding research funding directly affect how many resources are available for the billions of tiny tests, quality checks, and manufacturing innovations needed to bring the vaccines safely to market. In many ways, the future of personalized mRNA vaccines is as much about social commitment to scientific progress as it is about laboratory breakthroughs.
Potential Long-Term Benefits and Risks
- Benefits:
- Highly targeted cancer treatment with potentially fewer side effects.
- The ability to adapt and re-formulate treatments as tumors evolve.
- Enhanced overall survival and quality of life for patients.
- Opportunities to combine with other modern therapies, creating a multi-pronged attack against cancer.
- Risks:
- Unpredictable changes in tumor makeup may affect vaccine precision over time.
- Political and regulatory uncertainties could slow clinical deployment.
- Public mistrust or misinformation might undermine widespread vaccination campaigns.
- The high cost and technical demands of personalization could restrict access to only a few.
Balancing these potential benefits and risks will require a coordinated effort from researchers, policymakers, and the healthcare community. Open communication, unwavering support for scientific research, and a commitment to patient well-being are essential components of a future where personalized mRNA vaccines become a cornerstone of cancer treatment.
Looking Ahead: A Call for Unified Support and Continued Research
Despite the hurdles, the revolution of personalized mRNA vaccines against cancer continues to gain momentum. Researchers and clinicians are optimistic about the prospects, but they also recognize that the journey is loaded with challenges – from navigating little twists in immune responses to ensuring that the process is both scalable and affordable.
What remains clear is that this approach represents one of the most critical shifts in contemporary oncology. By equipping the immune system with the precise instructions needed to recognize and destroy cancer cells, personalized vaccines have the potential to make a significant impact on survival rates and quality of life.
To support this promising field, it is essential that public funding remains robust, regulatory frameworks stay science-based, and a clear line of communication is maintained between scientists, policymakers, and the broader public. Without these elements, the risk of stalling progress is real, and the lives of countless patients could be adversely affected.
Practical Steps for Advancing the Field
- Increased Federal Investment: Continued and expanded funding for cancer research is super important to maintain momentum.
- Enhanced Collaboration: Public-private partnerships can drive innovation and help streamline the complex issues inherent in personalized medicine.
- Public Education Campaigns: Clear, accessible information can help demystify the technology and reduce public hesitancy or misunderstanding.
- Robust Regulatory Support: Ensuring that regulatory frameworks are flexible yet rigorous will enable faster adaptation to new data and innovations.
The promise of personalized mRNA vaccines is not just a story of scientific triumph, but also a call to action for all stakeholders. It is a plea to support research and development, to embrace modern technology, and to back policies that allow such innovations to flourish. Only through a unifying commitment to science and patient care can we hope to see the full benefits of these life-saving treatments.
Conclusion: Embracing the Future of Cancer Care
The journey toward personalized mRNA vaccines for cancer is undeniably filled with twists and turns. From the meticulous process of tissue sampling and genetic sequencing to overcoming funding cuts and public skepticism, every step represents a critical piece of a larger puzzle. The work being done in laboratories, led by pioneers who are not afraid to dig into the confusing bits of cancer research, is laying a foundation for a future where treatment is truly individualized.
It is an exciting time to be part of this transformative era. The potential of mRNA technology, refined and proven during the COVID-19 pandemic, now extends into the realm of oncology. As we witness the early success of these vaccines in clinical trials and hear stories of patients regaining hope after years of illness, it becomes clear that the ability to tailor treatment to the genetic makeup of each tumor could redefine our approach to cancer care.
However, for this promise to be fully realized, society must work together. Enhanced funding, political support, and public trust are all necessary ingredients in the recipe for success. As stakeholders, we must not only celebrate the scientific breakthroughs but also add our voices to calls for continued investment and support in the fight against cancer.
In conclusion, personalized mRNA vaccines represent a paradigm shift in treating one of humanity’s most formidable adversaries. Their success could lead to a future where cancer is not seen as an inevitable death sentence but as a manageable condition with targeted, effective solutions. With the right support in place and a commitment to ongoing research, we may well be on the brink of a new era in cancer care – one where modern innovation and patient-centric approaches converge to save lives.
For patients, families, and healthcare providers alike, this innovative approach offers a beacon of hope. As the scientific community works through the tricky parts, tangled issues, and minor twists along the way, the battle against cancer may finally be tipped in our favor. It remains up to all of us, from legislators to everyday citizens, to champion and support these advancements for a healthier, brighter future.
Originally Post From https://www.scientificamerican.com/article/personalized-mrna-vaccines-will-revolutionize-cancer-treatment-if-federal/
Read more about this topic at
A step toward personalized immunotherapy for all
Personalized immunotherapy in cancer precision medicine