Nanocarriers in Cancer Therapy: An Opinion Editorial on Their Promise and Pitfalls
The realm of cancer treatment has witnessed some radical shifts over the past decade, and one of the most exciting fields making headlines is nanomedicine. Nanocarriers, those tiny vehicles engineered at the nanoscale, have shown remarkable promise for delivering anticancer drugs directly to tumor sites. By doing so, they help reduce unwanted side effects and improve drug efficiency. However, as with any cutting-edge technology, there remain a lot of tangled issues and complicated pieces to address.
In this editorial, I will take a closer look at the use of nanocarriers for cancer treatment. We will dive into the finer details of their design, the obstacles in clinical translation, and the exciting prospects for integrating them with immunotherapy. Along the way, we will figure a path through the nerve-racking challenges and subtle distinctions in effectiveness, safety, and sustainability in our fight against cancer.
Finding Your Way Through the Tricky Parts of Nanocarrier Technology
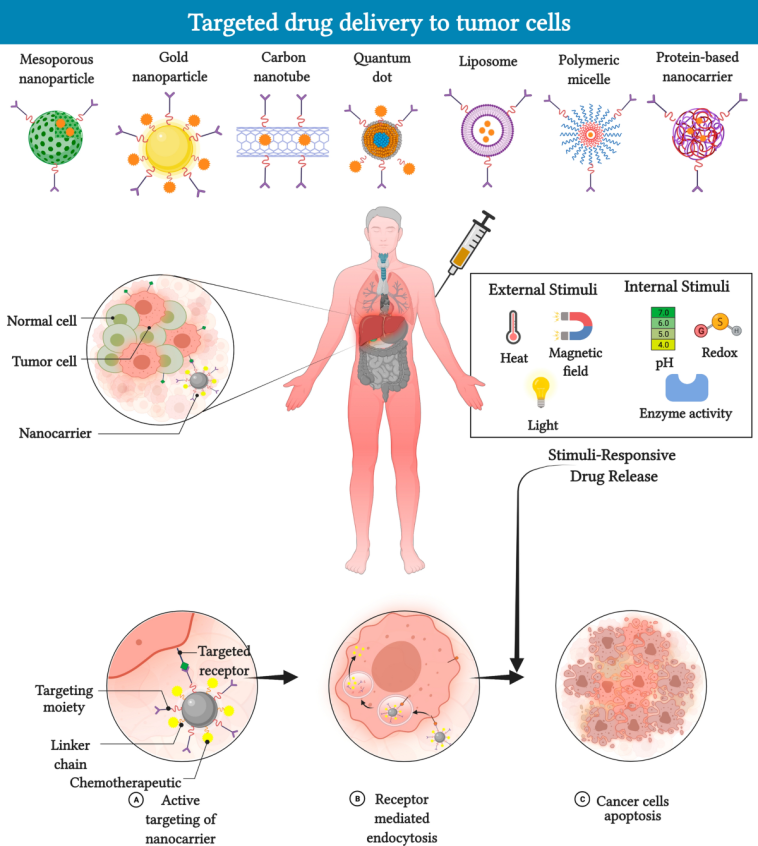
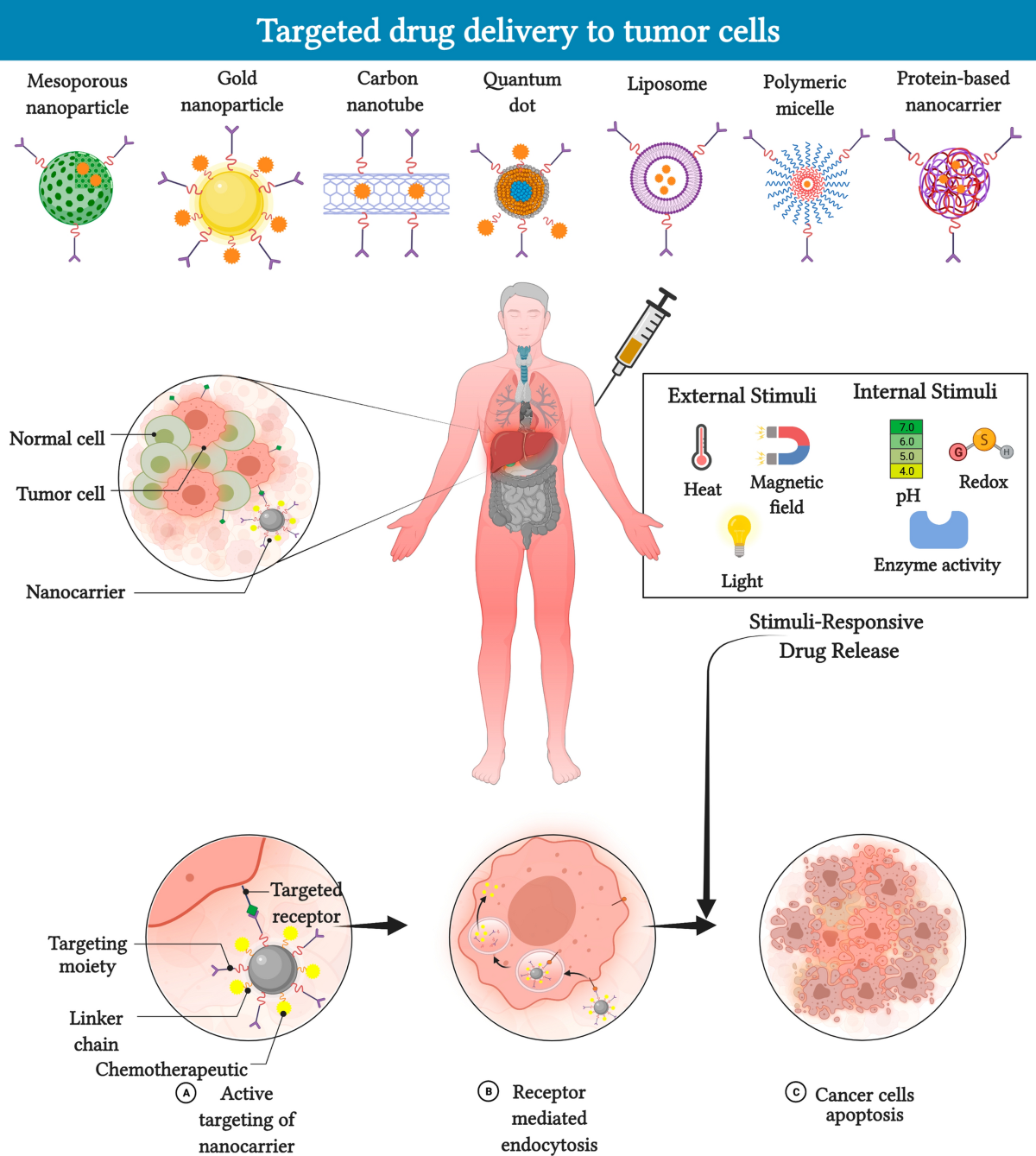
One of the most impressive aspects of nanocarrier-based drug delivery is its potential to precisely release drugs in the tumor microenvironment. Unlike conventional treatments, which often impact healthy tissues, nanocarriers can be engineered to respond to specific stimuli. They respond to pH differences, redox conditions, temperature variations, or even external cues such as light and ultrasound.
Yet, these sophisticated systems are not without their convoluted issues. There are several twists and turns in the design and development of these nanoscale devices. Here are a few of the critical challenges as well as shining points:
- Design and Synthesis: Creating a nano-delivery system involves figuring out the perfect material—be it polymers, liposomes, dendrimers, metal-based nanoparticles, or even hybrid constructs. The synthesis process is loaded with problems such as maintaining consistent size, shape, and surface properties. These parameters, while seemingly small, can have a huge impact on how the body identifies and clears these particles.
- Stability and Controlled Release: The ability to hold onto a therapeutic drug and then release it at the right moment in the tumor is a must-have feature. However, achieving a reliable, predictable release profile is a challenging bit. Factors like premature drug leakage or rapid clearance from the blood stream can hamper the intended outcomes.
- Targeting Specificity: Nanocarriers are often decorated with ligands that home in on particular receptors in cancer cells. Although these targeting strategies are critical, they sometimes fall short. The body’s natural defense systems may still clear these particles too quickly, or the complex blood environment might block the interactions between the carrier and its intended target.
- Scale-Up and Reproducibility: Moving from laboratory experiments to real-world production is another nerve-racking hurdle. Many of these systems work marvelously in controlled settings, but replicating them consistently in large batches for clinical use is often full of problems.
In summary, while the technology behind nanocarriers is groundbreaking, the tricky parts—ranging from synthesis consistency to controlled drug release—need ongoing refinement before these systems can be confidently implemented as standard cancer treatments.
Diving Into Drug Resistance: Overcoming the Tangled Issues of Cancer Treatment
Cancer drug resistance remains one of the most significant hurdles in oncology. Traditional chemotherapies often fail because cancer cells develop resistance mechanisms over time, leaving patients with limited options. Nanocarrier systems, with their sophisticated targeting and controlled release mechanisms, show enormous potential to bypass these resistant pathways.
When conventional treatments release a high dose of drugs systemically, cancer cells get the chance to adapt. In stark contrast, nanocarriers can encapsulate drugs and gradually deliver them, which may decrease the likelihood of the cancer cells building up resistance. They can also be designed to work around the overactive drug efflux pumps—those proteins that effectively pump drugs out of cells.
Some approaches to address these challenges include:
- Multifunctional Platforms: By combining various therapies—for instance, chemotherapy with gene therapy or photothermal treatment—nanocarriers can tackle the problem from multiple angles, making it harder for cancer cells to adapt.
- Stimuli-Responsive Systems: These carriers are programmed to release drugs when they detect a specific trigger, such as a drop in pH or an increase in temperature. Cancer tissues, which often have an acidic and/or hypoxic environment, provide the perfect conditions for such targeted release.
- Overcoming Efflux Pumps: Some nano-engineered systems have been designed to bypass cell membrane transporters. For example, by packaging the drug in a carrier that enters cells via endocytosis rather than passive diffusion, researchers can improve intracellular drug uptake.
These strategies are promising for making headway in the battle against drug resistance. They illustrate how nanocarriers can be turned from a mere drug transport mechanism into a dynamic tool that addresses some of the most overwhelming obstacles in modern oncology.
Understanding the Fine Points of Nanocarrier Design and Functionality
Engineering nanocarriers for cancer therapy is far from a one-size-fits-all approach. Each type—from liposomes and polymeric nanoparticles to dendrimers and metal-based systems—comes with its own set of fine points that dictate performance. To get into the nitty-gritty, here are some subtle parts of the design considerations:
- Size and Shape: The physical dimensions of nanocarriers are one of their most critical features. Nanoparticles that are too small may be filtered out by the kidneys, and those that are too large could be taken up by the spleen or liver. The optimal size range is often considered to be between 100 and 200 nanometers.
- Surface Chemistry and Charge: The exterior properties of nanocarriers influence how they interact with blood proteins and cell membranes. A slight difference in charge can determine whether a particle is stealthy enough to have a longer circulation time without being engulfed by the immune cells.
- Material Composition: The choice of material—be it biocompatible polymers, lipid mixtures, or inorganic metals—affects both the therapeutic potential and toxicity profile. For instance, liposomes are known for their biocompatibility, whereas some inorganic nanoparticles may be prone to bioaccumulation.
- Functionalization with Ligands: Decorating nanoparticles with targeting molecules such as antibodies, peptides, or small molecules is key to precise delivery. Nonetheless, these modifications sometimes become off-putting if they lead to immune recognition or rapid clearance.
Constructing nano-delivery systems is as much an art as it is science. Researchers must cleverly balance the need for effective drug loading, stable circulation profiles, and precise release mechanisms all while keeping the overall system biocompatible and scalable for clinical translation. The little details matter—a slight alteration in polymer composition or ligand density can result in a dramatically different outcome in vivo.
Working Through the Tense Challenges of Clinical Translation
Despite the impressive lab-based performance of many nanocarriers, the move to clinical practice is often loaded with problems. Translating a promising concept into a widely accepted cancer treatment is a multifaceted process marked by several nerve-racking challenges:
- Safety and Toxicity: One of the predominant concerns is whether these tiny carriers will trigger unintended immune responses or accumulate in non-target organs. Even when designed with biocompatible materials, the unpredictable behavior of nanoparticles in the human body demands thorough long-term studies.
- Production and Scalability: Laboratory-scale production methods rarely account for the tangled issues that come with mass production. Reproducibility and consistency across production batches are essential for regulatory approval and eventual clinical use.
- Regulatory Hurdles: With so many moving parts—each nano-device’s feature like size, surface coating, and drug release profile—the task of compiling comprehensive, standardized safety data is overwhelming. Regulatory bodies require detailed information about the pharmacokinetics, biodistribution, and clearance of these systems.
- Cost and Complexity: The synthesis methods and materials used in nanocarrier systems can often be expensive and complicated, making it challenging to produce them at a reasonable cost for widespread clinical use.
These issues underscore why the journey from bench to bedside is anything but straightforward. However, the potential payoffs in precision treatment and reduced systemic toxicity make it an area worthy of continued research and investment.
Integrating Immunotherapy with Smart Nanocarriers: Enhancing Cancer Treatment
Another exciting frontier is the merger of nanocarrier technology with cancer immunotherapy—a field that aims to harness the body’s own immune system to fight tumors. The idea here is to use nanoparticles not only to deliver cytotoxic drugs but also to transport immune-stimulating agents directly to the tumor microenvironment.
Immunotherapy has already revolutionized cancer treatment, but its full potential is often limited by factors like poor penetration into tumor tissue and widespread off-target effects. Smart nanocarriers can help overcome these limitations. For instance, by co-loading a chemotherapeutic agent along with an immune checkpoint inhibitor, these carriers can both kill cancer cells directly and prime the local immune attack.
Some notable benefits of combining nanocarriers with immunotherapy include:
- Enhanced Targeting: Nanocarriers can be designed to home in on immune cells such as dendritic cells, which then kick-start an immune response. They can also be engineered to mimic immune cell membranes, helping them blend into the biological landscape more easily.
- Reduced Systemic Side Effects: By focusing the delivery of immunomodulatory agents on the tumor site, these systems help avoid the off-target activation of immune cells, which can often lead to severe inflammatory responses.
- Synergistic Effects: The combination of direct cancer cell killing with immune system activation has been shown to produce better overall outcomes, reducing the chances of relapse and metastasis.
This approach underscores how nanocarrier systems can be used as versatile platforms—not just for straightforward drug delivery but for multi-functional, combination treatments that provide a more comprehensive attack on cancer.
Addressing the Hidden Complexities of Theranostic Nanoplatforms
Theranostics—the combination of therapy and diagnostics—is an emerging concept in cancer treatment that capitalizes on the capabilities of nanocarriers. Imagine a system that not only delivers a drug in a controlled manner but also helps monitor its progress in real time through imaging techniques. This dual functionality is incredibly appealing as it allows doctors to get immediate feedback about treatment effectiveness.
Theranostic nanoplatforms often incorporate both a therapeutic payload and a contrast agent that can be visualized via MRI, PET, or fluorescence imaging. The hidden complexities of these systems include:
- Dual-Function Integration: Combining two functionalities—therapy and imaging—into one single platform is a tricky part. The materials and synthesis methods must support both roles without interfering with each other.
- Tuning Release and Signal Strength: The release kinetics of the drug and the imaging signal need to be well synchronized. Any slight mismatch can lead to misinterpretation of treatment progress.
- Biocompatibility Challenges: Adding an imaging component, such as a heavy metal, can sometimes increase toxicity risks. It is crucial that these modifications maintain the safety profile of the overall system.
Despite these complications, theranostic nanoplatforms represent a key step toward personalized medicine. They offer the possibility of adapting treatment protocols in real time by confirming whether the therapeutic agent has reached the target site and is functioning as planned.
Smart, Stimuli-Responsive Nanocarriers: The Future of Personalized Cancer Therapy
One of the most promising innovations on the horizon is the development of smart, stimuli-responsive nanocarriers. These systems are equipped to release their cargo only when they detect a specific trigger in the tumor microenvironment. For example, many tumors feature an acidic extracellular pH and elevated levels of glutathione—a combination that can be used to activate the release of the drug precisely where it is needed.
Let’s break down some key stimuli and the advantages associated with them:
- pH-Responsive Release: Tumor tissues often have a lower pH compared to healthy tissues. Nanocarriers designed to react to these pH changes can ensure that the drug is released primarily in the cancer site.
- Redox-Responsive Systems: Elevated levels of reducing agents such as glutathione in cancer cells can trigger the disintegration of certain bonds in the nanocarrier, liberating the encapsulated therapeutic in a controlled fashion.
- Light and Ultrasound Activation: External stimuli like near-infrared (NIR) light or ultrasound not only allow doctors to see where the nanoparticle is but also to give a precise “on/off” command for drug release. This level of control is especially useful in combination therapies, where timing can make the difference between synergy and toxicity.
- Magnetic Field Guidance: Magnetic nanoparticles can be steered using an external magnet, ensuring that they accumulate at the tumor site. After localization, the release can be designed to occur under another stimulus.
These responsive systems are key to creating personalized treatment protocols that adapt to the patient’s individual tumor characteristics. They represent a must-have development, especially considering the overwhelming challenge of tailoring treatments to the subtle differences between tumors in different patients.
Steering Through the Economic and Regulatory Maze
Even if the scientific and technological hurdles are overcome, there are still plenty of confusing bits when it comes to getting nanocarrier-based therapies into the clinic. Regulatory approval is a particularly intimidating and loaded process because of the following reasons:
- Comprehensive Preclinical Data: Due to the fine shades in how these particles behave in the body, regulators require extensive data on biodistribution, pharmacokinetics, and long-term toxicity.
- Complex Manufacturing Processes: Scaling up nanocarrier production while maintaining consistent quality is a tricky part. Each batch must meet strict specifications, and minor variations in synthesis can lead to significant changes in product performance.
- High Costs of Development: Developing these advanced systems is expensive, and the cost can become nerve-racking for companies with limited budgets. Despite the promising science, stakeholders must carefully balance research investment with the predicted return.
Tables or charts that detail the pros and cons, as well as a roadmap for regulatory requirements, are extremely useful tools to help both researchers and industry players get around these challenges. Although it might feel like a maze full of confusing bits, coordinated efforts between academic researchers, pharmaceutical companies, and regulatory bodies are essential to bringing these promising therapies to patients.
What Does the Future Hold for Cancer Nanomedicine?
Looking ahead, the future of nanocarrier systems in cancer therapy appears bright but is also paved with challenges that need to be managed carefully. Here are some of the key trends and prospects that I find super important:
- Personalized Medicine: Advances in genomics and proteomics are set to make treatments more patient-specific. Nanocarriers could be tailored to deliver drugs based on a tumor’s unique genetic profile, ensuring that the treatment is as effective as possible.
- Combination Therapies: The ability to deliver multiple therapeutic agents simultaneously—such as combining chemotherapeutic drugs with gene-silencing molecules or immune modulators—allows for tackling cancer from multiple angles.
- Theranostic Platforms: As discussed earlier, the integration of diagnostic imaging with therapeutic delivery will revolutionize treatment monitoring and personalized adjustments.
- Bio-Inspired Designs: Recent trends include using cell membrane coatings on nanocarriers to help them evade the immune system, thereby prolonging their circulation time and enhancing targeting capabilities.
- Sustainable Production: Future research must also focus on developing eco-friendly and cost-effective manufacturing processes. This is critical for making these therapies widely accessible in clinical settings.
This vibrant intersection of technology, biology, and engineering promises a new era where treatments are not only more effective but also come with fewer side effects. Despite the overwhelming challenges and problematic regulatory environments, the progress made so far makes me optimistic that nanocarrier systems will become a cornerstone of cancer therapy in the not-too-distant future.
Expert Opinions and Community Perspectives
It is important to note that opinions differ among experts in the field, and while many celebrate the breakthroughs, others are cautious, pointing to the nerve-racking issues in reproducibility and patient safety. In research circles, there is agreement that:
- The delicate balance between drug efficacy and patient safety remains a key focus area.
- More standardized protocols are needed to reduce the fine differences seen from one batch of nanocarriers to another.
- Collaborative efforts across disciplines—combining materials science, biology, and clinical research—are essential to push the envelope further.
These community insights underline that while the science is promising, the real-world application of these advanced systems is still in the early days, and one must remain both hopeful and pragmatic.
Wrapping Up: The Promising Path Ahead
In summary, nanocarrier technologies represent a groundbreaking leap forward in delivering targeted cancer therapies. They offer the potential to reduce systemic toxicity, overcome drug resistance, and even merge treatment with diagnostic imaging. However, as we have seen, there are a multitude of tangled issues and complicated pieces—from the fine details of nanoparticle design to the overwhelming challenges of clinical translation—that must be managed with care.
From my perspective, the future for nanomedicine is incredibly promising if the field continues to work through the tricky parts. With sustained investment in research, more collaborative efforts between academia and industry, and a will to address the subtle differences that can affect outcomes, we have every reason to be optimistic. Smart, stimuli-responsive nanocarriers, in particular, have the potential to transform personalized medicine in oncology, providing treatments that are both more effective and only released when absolutely needed.
The road ahead is certainly loaded with problems, and many of the nervous, off-putting challenges will require creative and innovative solutions. Yet, if we can steer through these twists and turns by focusing on both the science and the practical aspects of product development, we may very well see a future where cancer treatments are not only more successful but also significantly kinder to patients.
Ultimately, the promise of nanocarriers lies in their ability to combine multiple treatment modalities into one finely tuned system. Whether it is through the use of bio-inspired coatings that help particles evade our immune defenses, or the integration of imaging agents that allow clinicians to track therapy in real time, these smart systems are destined to play a critical role in the next generation of cancer care.
As researchers continue to dig into and refine the hidden complexities of these systems, the hope is that nanocarrier-based therapies will move from the laboratory to the clinic—making that daunting leap into routine medical practice. It is a journey full of convoluted details and overwhelming challenges, yet it is one that carries with it the tremendous potential to redefine how we treat one of the world’s most formidable diseases.
In conclusion, while there is still much work to be done, the evolution of nanocarriers in cancer therapy is an essential development that deserves our continued attention. With the promise of increased precision, reduced side effects, and enhanced therapeutic efficacy, this innovative field is poised to pave the way for a brighter, more personalized future in cancer treatment.
For patients, clinicians, and researchers alike, keeping a close eye on these developments and fostering interdisciplinary collaborations will be key to unlocking the full potential of nano-enabled oncology treatments—a challenge that is as immense as it is exciting.
Originally Post From https://www.dovepress.com/current-advances-in-nanocarriers-for-cancer-therapy-peer-reviewed-fulltext-article-IJN
Read more about this topic at
Smart nanocarrier-based drug delivery systems for cancer …
Smart nanoparticles for cancer therapy