An Opinion on Postoperative ctDNA Clearance and Its Impact on Colorectal Cancer Treatment
The oncology community has long sought reliable markers to indicate the success of surgery and subsequent therapies in colorectal cancer (CRC). Recent findings from the INTERCEPT study, presented at the European Society for Medical Oncology Congress 2025, offer promising insights into the role of circulating tumor DNA (ctDNA) clearance after surgery and adjuvant therapy. In this opinion editorial, we take a closer look at the study’s findings, the implications for treatment strategies, and the challenges in interpreting these results.
Understanding the Role of ctDNA in Postoperative Patient Monitoring
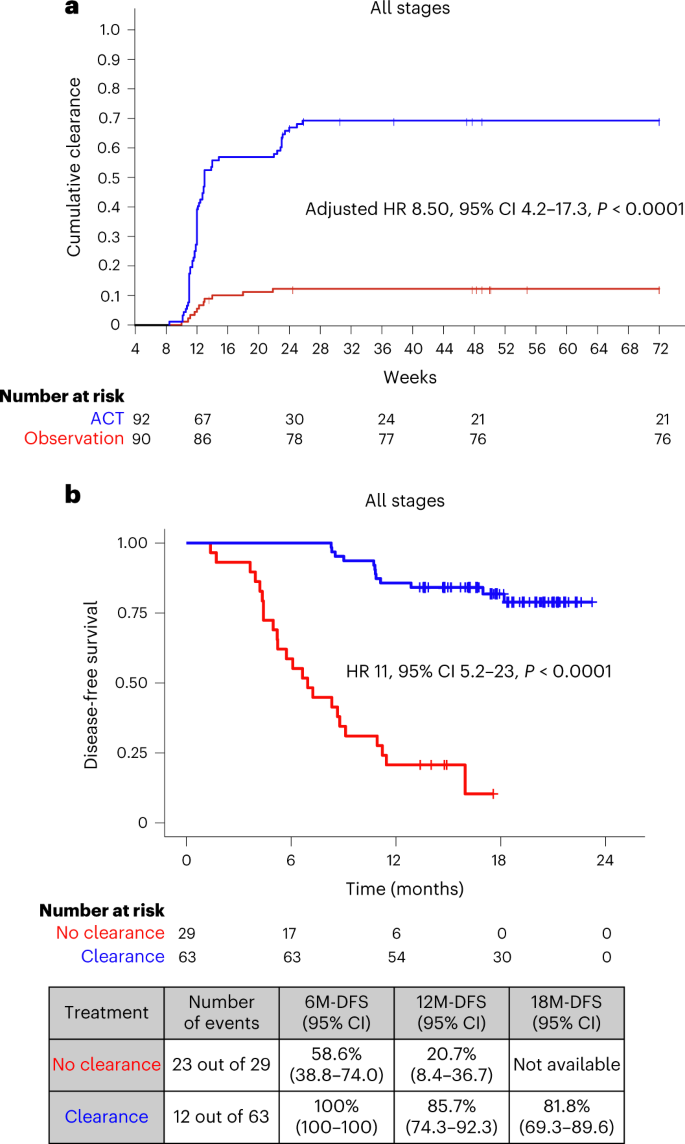
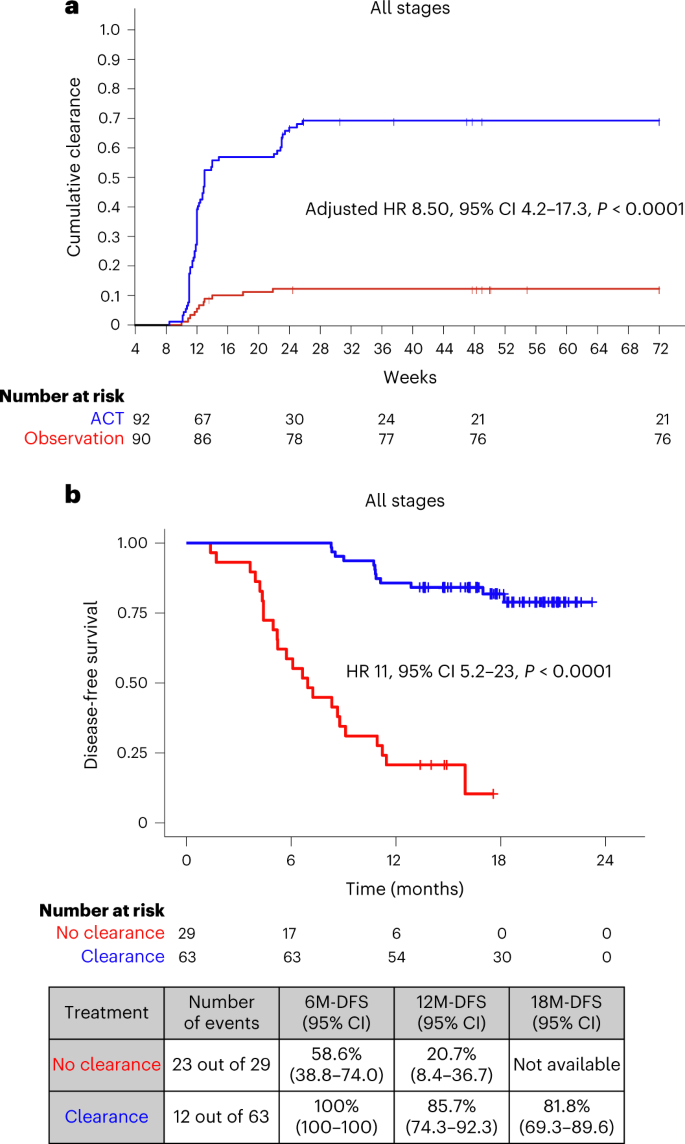
Circulating tumor DNA has emerged as a potential tool for monitoring minimal residual disease in patients following curative-intent procedures. In simple terms, ctDNA is a biomarker that may help indicate whether cancer cells persist after surgery. Studies have shown that the presence of ctDNA postoperatively is a strong predictor for disease recurrence. The INTERCEPT study further reinforces the notion that adjuvant therapy can facilitate ctDNA clearance in a notable subset of patients, suggesting that negative ctDNA results can be a sign of improved disease-free survival (DFS).
When looking at the role of ctDNA, it is important to recognize that this marker addresses several tricky parts of postoperative management:
- Diagnosing minimal residual disease without invasive procedures.
- Predicting recurrence early so that timely interventions can be made.
- Monitoring patient response to adjuvant therapies in real time.
This issue, although promising, is not without its tangled issues. Although ctDNA clearance is an essential indicator, interpreting fluctuations in ctDNA levels may involve confusing bits that require more exploration and discussion.
Adjuvant Therapy: How Does It Benefit Colorectal Cancer Patients?
Adjuvant treatments, which are administered in the period following surgical resection, have historically been used to eradicate any remaining cancer cells. One of the significant observations from the INTERCEPT study is that adjuvant therapy is associated with ctDNA clearance in approximately 26% of postoperative patients who initially tested positive for ctDNA. Moreover, this ptient group experienced superior DFS outcomes when clearance was observed over at least two tests.
The study’s analysis provides confidence that adjuvant therapy does more than just serve as a backup measure—it may have a critical role in reducing tumor molecule levels that are visible in patient blood. Here are some key takeaways:
- Clearance Rate: Around a quarter of the patients who tested positive for ctDNA after surgery showed clearance following adjuvant therapy.
- Consistency in Testing: Patients demonstrating clearance on at least two sequential tests had markedly improved DFS outcomes.
- Stage-wise Benefit: Both early-stage (I-III) and stage IV patients showed significant benefits, creating a broader context for utilizing adjuvant therapy beyond traditional high-risk groups.
Despite these positive outcomes, there remain some nerve-racking uncertainties. The durability of spontaneous ctDNA clearance—that is, clearance occurring without any additional intervention—appears to be very low. This raises further questions on the fine points of managing patient expectations and tailoring treatment protocols.
Decoding the INTERCEPT Study Data
The INTERCEPT study enrolled 1,301 patients with newly diagnosed or previously treated resectable stage I-IV CRC. With 53% of patients in stage I-III and 47% in stage IV, the study provided a broad insight into various patient populations. Routine surveillance through imaging and laboratory tests, coupled with ctDNA assay testing, allowed researchers to observe the dynamics of ctDNA over time. Notably:
- 69% of patients had ctDNA negativity on all tests conducted after surgery.
- 18% remained ctDNA positive on all tests, indicating persistent disease activity.
- A small percentage (about 11.5%) showed conversion from ctDNA positivity to clearance in one or more tests—an encouraging sign when observed consistently.
When analyzing test outcomes, it is helpful to think of the results as a table that summarizes patient responses:
| ctDNA Status | Percentage of Patients | Notable Findings |
|---|---|---|
| Consistent Negative Results | 69% | Strong correlation with better DFS. |
| Consistent Positive Results | 18% | Indicative of persistent minimal residual disease. |
| Clearance on ≥2 Tests | Approximately 3.1% (post-surgery), 13 (17%) in ctDNA-positive patients post-adjuvant therapy | Critical threshold for significantly improved DFS outcomes. |
| Conversion from Negative to Positive | 8% (overall) and 9% (post-adjuvant therapy) | Highlights the unpredictable nature of ctDNA levels. |
The table above provides a simple framework to understand the distribution and significance of ctDNA findings. It is clear that while a majority of patients experience negative ctDNA results post-surgery, a sizeable minority remain positive, thus risking potential relapse without effective intervention.
Unpacking the Tricky Parts of ctDNA Testing and Data Interpretation
While the data seems encouraging, there are several tangled issues when incorporating ctDNA testing into routine clinical practice. A few of the common challenges include:
- Test Variability: Differences in ctDNA assay sensitivity can lead to small distinctions in reported levels. These slight differences may impact clinical decision-making.
- Timing of Testing: Determining the optimal intervals for ctDNA monitoring—every three months as suggested in the study—requires careful planning. Missing the right window might lead to misinterpretation of the patient’s actual status.
- Interference from Spontaneous Clearance: A small group of patients showed spontaneous clearance without any additional treatment, making it tricky to attribute such outcomes solely to adjuvant therapy.
- Technical Hurdles: The nuances of ctDNA measurement require highly standardized laboratory protocols. Variability between testing centers may add to the complicated pieces of ensuring consistent results.
These points underscore that while ctDNA is a super important marker, using it effectively involves untangling a variety of practical and technical challenges. It is akin to finding your way through a maze of subtle details and unpredictable test outcomes.
Clinical Implications: Beyond the Numbers
For clinicians and patients alike, the bottom line remains that postoperative ctDNA clearance can offer a window into long-term treatment success. The results from the INTERCEPT study hint at a tailored approach where patient treatment could be adjusted based on ctDNA dynamics. Consider these clinical takeaways:
- Personalized Treatment: By regularly monitoring ctDNA levels, oncologists can better steer through treatment modifications in real time. Patients demonstrating persistent ctDNA positivity may benefit from additional therapeutic interventions to mitigate the risk of recurrence.
- Enhanced Surveillance: Integrating ctDNA monitoring into standard follow-up protocols can provide earlier signals of relapse. This proactive approach may be particularly useful in managing both early-stage and metastatic CRC cases.
- Risk Stratification: Patients who show clearance on more than one test tend to have a more favorable prognosis. These findings can help surgeons and medical oncologists figure a path to reducing overtreatment in low-risk cases.
- Patient Reassurance: Clearly communicated ctDNA monitoring results can help alleviate some of the anxiety associated with postoperative uncertainty. Knowing that a negative ctDNA test is associated with better outcomes may help patients feel more confident about their recovery journey.
Yet, while these implications are promising, the challenges associated with standardizing testing methodologies remain an area needing further development. Even with the best technology, there is still a need for consensus on how to interpret low-level positivity versus complete negativity.
Considering the Broader Picture: Alternative Approaches and Nutrition
Beyond traditional treatments, the evolving field of alternative medicine and comprehensive patient care provides context for the importance of biomarkers like ctDNA. A holistic approach in treating CRC also considers nutrition, lifestyle adjustments, and supportive care techniques. Integrating these aspects can help manage the overall well-being of a patient in ways that are linked to improved treatment outcomes.
For example, clinicians are increasingly recommending that patients adopt dietary changes, such as increased fiber intake or a reduced intake of processed foods, to support recovery and bolster the immune system after surgery. Coupled with advanced monitoring techniques such as ctDNA assays, these measures represent multitiered efforts to ensure comprehensive care.
Some therapeutic strategies that align with these approaches include:
- Integrative Nutrition Plans: Guiding patients toward diets rich in antioxidants and anti-inflammatory foods as a complementary approach to adjuvant therapy.
- Holistic Support Services: Incorporating stress-reduction practices, like yoga or meditation, which may indirectly support the biological efficacy of therapy.
- Regular Follow-Up Evaluations: Using both traditional imaging and advanced laboratory assessments such as ctDNA testing to form a complete picture of patient health.
While these strategies do not replace the need for rigorous clinical treatment, they represent key components in elongating disease-free periods and improving quality of life. Integrating the patient’s lifestyle into the larger treatment plan is as essential as the surgical or pharmaceutical interventions themselves.
The Challenges of Implementing ctDNA Testing in Everyday Practice
Although ctDNA testing appears promising, its integration into daily clinical practice is not without challenges. Many oncologists must first tackle several intimidating issues, including:
- Cost and Accessibility: Not every healthcare facility has the resources to perform sensitive ctDNA assays. Ensuring equitable access is a critical hurdle to overcome.
- Standardization Across Laboratories: Differences in test platforms and protocols may lead to variable results. Standardization is super important to ensure that results are directly comparable across different settings and patient groups.
- Interdisciplinary Communication: Surgeons, oncologists, pathologists, and laboratory experts all need to work closely to interpret ctDNA findings correctly. The collaboration between these stakeholders is key to managing the patient’s course effectively.
- Patient Education: Explaining the significance of ctDNA levels, the scheduling of tests, and how results might affect treatment involves intricate conversation and clear communication. Simplifying these complex ideas for patients without diminishing their importance is essential.
Addressing these hurdles will require not only further clinical research but also a systemic change in how cancer care is delivered. Making ctDNA a routine part of surveillance protocols involves concerted effort and clarity about how these markers should be interpreted in the context of individual patient histories and treatment responses.
Future Directions: Integrating ctDNA in Personalized Cancer Treatment
The potential applications of ctDNA are far reaching. Beyond its immediate implications for monitoring treatment response and recurrence risk in CRC, there are broader prospects for personalized cancer care. The idea is that, eventually, ctDNA dynamics could become a standard part of decision-making processes for many types of solid tumors.
Future research should focus on several key areas:
- Longitudinal Studies: More long-term studies are needed to understand the implications of persistent low-level ctDNA positivity and the timeframe over which tumor DNA levels change post-surgery.
- Comparative Effectiveness of Assays: Determining which platforms offer the most reliable and reproducible results would help clinicians choose the best test for their patients.
- Defining Optimal Testing Intervals: Establishing standard protocols around the timing of ctDNA assessments could reduce the confusing bits associated with variable testing schedules.
- Integrative Biomarker Panels: Combining ctDNA with other biomarkers and imaging findings may offer a more comprehensive picture of tumor biology, thereby fine-tuning decisions about adjuvant therapy and surveillance strategies.
As researchers work on these issues, both patients and clinicians can remain hopeful that the integration of ctDNA testing into routine practice will lead to more personalized and effective cancer treatment protocols. In this complex field marked by both promise and many twists and turns, each new discovery brings us one step closer to a future where cancer is managed through a blend of precise diagnostics and tailored therapies.
Understanding Patient Perspectives in the Era of ctDNA Testing
For patients, the interplay between advanced diagnostics and traditional treatment approaches can be both reassuring and overwhelming. While many welcome the possibility of a biomarker that can signal their risk of relapse, others are anxious about what a positive ctDNA result might mean for their long-term outcomes. Clear communication is therefore a must-have for clinicians, who are tasked with making these technical results understandable to patients.
Consider a few patient-centered approaches:
- Transparent Communication: Explain in simple terms how ctDNA testing works, what the results imply, and the limitations of the current knowledge.
- Shared Decision-Making: Involve patients in the decision-making process, particularly when considering additional treatments for those who remain ctDNA positive.
- Psycho-Social Support: Provide counseling and support services to help patients navigate the nerve-racking bits of living with cancer and its often unpredictable course.
- Educational Resources: Offer accessible materials that break down the scientific details into everyday language, helping patients anticipate how changes in their ctDNA levels might guide treatment.
When patients are well-informed, they are better positioned to participate actively in their care, which can improve overall satisfaction and, potentially, outcomes. The role of ctDNA testing, therefore, extends beyond the confines of labs and research centers—it also touches the human element of trust, hope, and collaboration between patients and healthcare providers.
Weighing the Benefits Against the Challenges
Like many new developments in medicine, ctDNA testing presents us with both significant promise and certain tricky parts. To summarize, the potential benefits include:
- Earlier detection of recurrence, potentially leading to more timely interventions.
- The ability to tailor adjuvant treatments based on real-time feedback from the patient’s biology.
- Enhanced patient reassurance when ctDNA clearance correlates with better disease-free survival.
- A step toward truly personalized oncology, where treatment protocols are fine-tuned based on individual molecular responses.
On the other hand, clinicians and researchers must address several tangled issues:
- Ensuring that ctDNA testing methods are standardized and reproducible across different centers.
- Clarifying the precise impact of low-level ctDNA positivity, which may vary between patients with different stages of cancer.
- Addressing the added cost and resource requirements while ensuring that benefits justify the investment.
- Developing clear patient education programs to explain what the test results mean in everyday language.
Balancing these pros and cons requires ongoing research, collaboration across different areas of medicine, and a commitment to patient-centered care. Ultimately, building consistent guidelines for the use of ctDNA as a biomarker will help both providers and patients work through the many tricky parts of post-treatment cancer management.
Concluding Thoughts: Toward a More Tailored Oncology Future
The INTERCEPT study’s findings mark an important milestone in our understanding of how adjuvant therapy can help clear ctDNA in patients with CRC—a signal that is closely linked with improved disease-free survival. While the road ahead is filled with some intimidating and complicated pieces, the study sparks hope that we may soon refine our treatment strategies even further.
By integrating ctDNA testing into everyday clinical practice, oncologists can not only find their way through the confusing bits of postoperative management but also figure a path toward more personalized cancer care overall. The challenge now lies in standardizing testing procedures, expanding long-term data, and effectively communicating these technical details to patients in a straightforward manner.
As we take a closer look at these new developments, it becomes clear that ctDNA is not just another laboratory variable—it is a potential cornerstone in the multidisciplinary approach to oncology. With proper research and collaboration, we can harness this marker to improve patient outcomes, refine our treatment protocols, and ultimately, transform the patient journey in colorectal cancer treatment.
In conclusion, the adoption of ctDNA clearance as a measure of therapeutic success reflects a broader shift in oncology toward personalized, data-informed cancer care. This evolution, marked by its twists and turns, reminds us that every small step forward not only informs our current practice but also paves the way for future innovations. For patients and clinicians alike, the promise of ctDNA testing lies in its potential to bring clarity to a previously uncertain post-surgical landscape, providing both reassurance and a clearer direction for ongoing treatment.
As the integration of ctDNA within clinical protocols advances, collaborative efforts among oncologists, lab technicians, and researchers will remain essential. It is through this integrated approach—combining modern technology, rigorous testing, and compassionate patient care—that we can truly harness the super important insights offered by ctDNA dynamics in the battle against colorectal cancer.
Only by working through every twisted issue and paying attention to each subtle detail can we hope to achieve the ultimate goal: more effective, tailored treatments that not only extend survival but also improve quality of life for every patient facing colorectal cancer.
Originally Post From https://www.cancernetwork.com/view/adjuvant-therapy-confers-postoperative-ctdna-clearance-dfs-benefit-in-crc
Read more about this topic at
Adjuvant Therapy Confers Postoperative ctDNA Clearance …
The emerging clinical utility of ctDNA to guide adjuvant …