Emerging Treatment Strategies in Lung Cancer: A Modern Perspective
The landscape of lung cancer treatment continues to evolve, bringing both exciting breakthroughs and challenging twists and turns for clinicians and patients alike. In recent trials, researchers have been testing new combinations of therapies for small cell lung cancer (SCLC) and non–small cell lung cancer (NSCLC), offering hope but also raising tricky parts that demand close attention. In this opinion editorial, we take a closer look at the modern challenges in immunotherapy, chemoradiation, and preventive brain irradiation, as well as a new era of personalized medicine using liquid biopsies. We will explore the many layers of clinical data, discuss the areas loaded with problems, and consider the emerging approaches shaping the future of lung cancer care.
Exploring the Combined Use of Immunotherapy and Chemoradiation
Recent clinical trials have unveiled surprising results regarding the sequential administration of chemoradiotherapy and immunotherapy. This emerging strategy was initially seen as a promising method to improve patient outcomes by harnessing the body’s immune system to fight cancer once the main tumor burden was targeted by traditional chemoradiation. However, this approach comes with several tricky parts that must be considered when deciding the best course of action for lung cancer patients.
Understanding the Data from Phase 3 Trials
Data from the phase 3 NRG-LU005 trial (NCT03811002) delivered some unexpected findings: adding immuno-oncology (IO) agents, such as atezolizumab (Tecentriq), concurrently with chemoradiotherapy did not present a survival benefit for patients with limited-stage SCLC (LS-SCLC) when compared to chemoradiotherapy alone. With a 3-year overall survival rate of 44.7% in the immunotherapy arm versus 50.3% in the control, the results have prompted many experts to reconsider the timing and combination of these powerful drugs.
Concurrent Versus Consolidative Treatment: Weighing the Options
The findings suggest that administering immunotherapy during chemoradiation might inadvertently negate the beneficial effects of either treatment when administered concurrently. One theory is that radiation may cause lymphodepletion in the thoracic region, thereby depleting the very white blood cells needed to mount a robust immune response. As a result, the timing of immunotherapy becomes a critical factor. Instead of concurrent administration, many experts now favor a consolidative approach: providing immunotherapy only after completing chemoradiation, as long as imaging shows no progression of the disease.
Rethinking Prophylactic Cranial Irradiation (PCI) in SCLC
Prophylactic cranial irradiation (PCI) has long been a cornerstone in the treatment of SCLC. Its goal is to prevent the spread of cancer to the brain, a sanctuary site for metastatic cells. Nonetheless, recent trial outcomes have introduced a tangled issue regarding its true benefit in extensive-stage SCLC (ES-SCLC).
Historical Context and Recent Challenges
Historically, early studies and cooperative group efforts supported PCI for both limited-stage and extensive-stage lung cancer cases, driven by its ability to decrease intracranial metastases and potentially improve survival. However, in 2017, a pivotal Japanese phase 3 trial (UMIN000001755) called this longstanding belief into question by showing that PCI offered no survival benefit in patients with extensive-stage disease. These findings have led many to re-evaluate the risks and benefits of PCI.
Assessing the Risks and Benefits for Modern Patients
When deciding whether to administer PCI in the modern era, physicians must consider not only the potential survival benefits but also the acute and long-term toxicities associated with radiation treatment. These include cognitive deficits and other negative impacts on quality of life. With advances in imaging, such as baseline and every-three-month magnetic resonance imaging (MRI) scans, some argue that regular monitoring might be a sensible alternative to the blanket use of PCI, especially when it might contribute to overwhelming, off-putting side effects.
Personalizing Lung Cancer Treatment With Liquid Biopsies
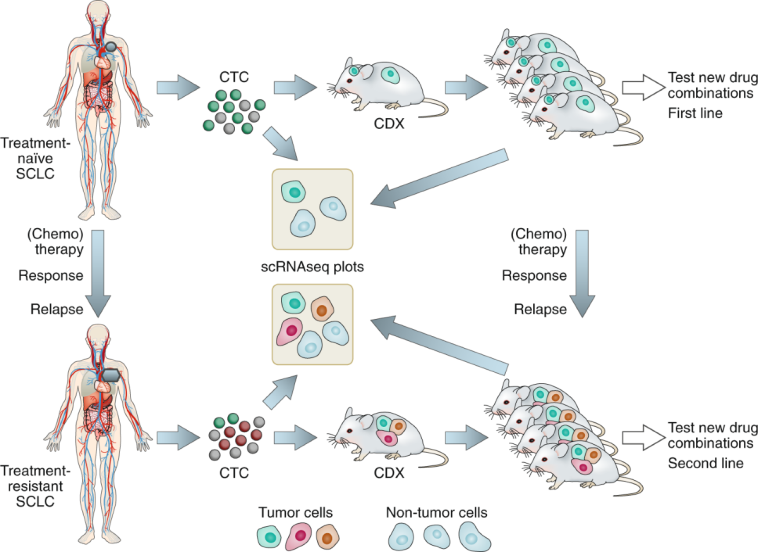
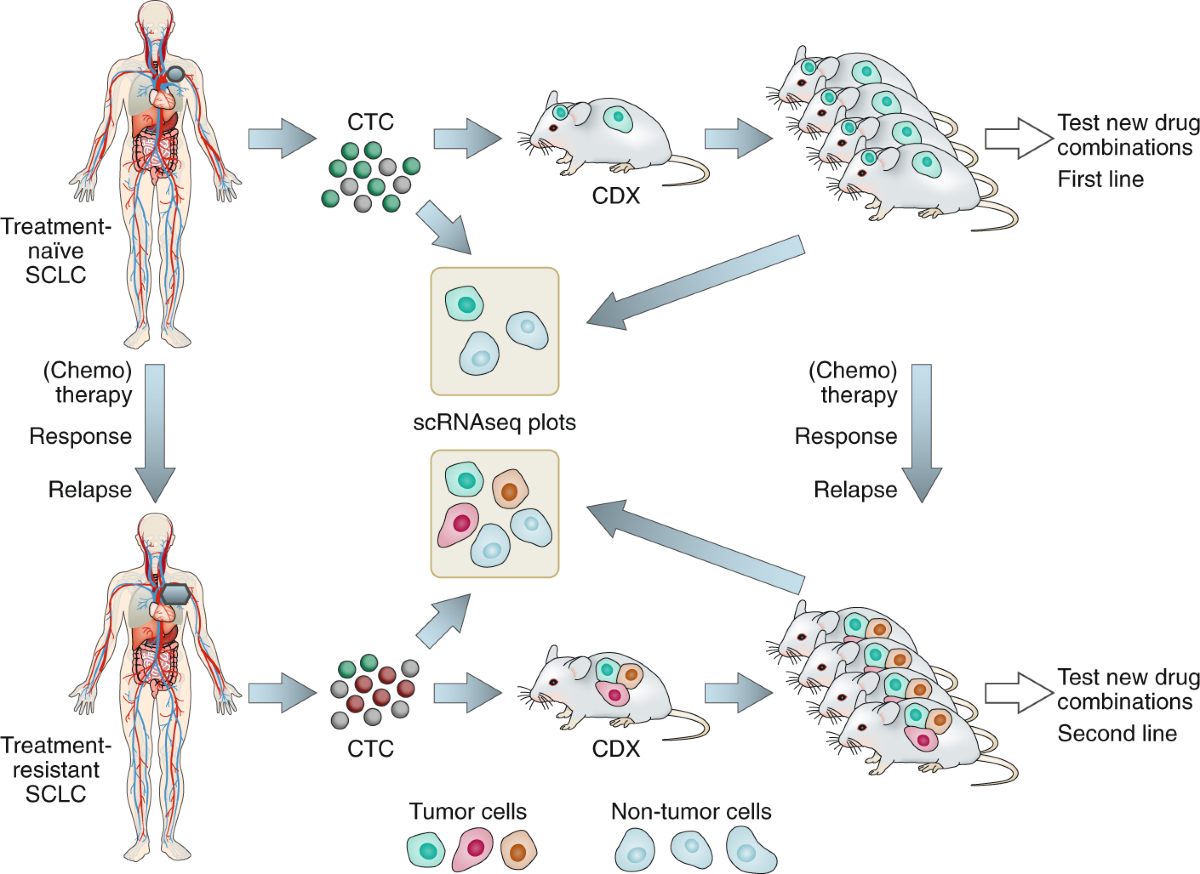
One of the key trends in modern oncology is the move toward personalized medicine. At the Indiana University School of Medicine, translational research led by experts like Dr. Misty D. Shields is exploring longitudinal liquid biopsies as a means to track tumor changes over time. This innovation represents a significant step forward in making treatment decisions more tailored and dynamic.
How Liquid Biopsies Enhance Personalized Care
Liquid biopsies involve regular collection of blood samples to study RNA expression, DNA mutations, methylation patterns, and proteomic profiles. This method allows researchers to get a closer look at how a patient’s tumor evolves throughout treatment, pinpointing novel mutations or subtypes that might predict a patient’s response to certain drugs. Such fine points and subtle parts of tumor biology are critical in adapting treatment at every step, especially in relapsed SCLC where the tumor’s profile might change rapidly.
Keeping Track of Tumor Changes Over Time
The longitudinal design of these studies means that blood is collected at multiple time points—often matching imaging intervals that can range from six weeks to three months. This iterative process allows oncologists to gather the nitty-gritty details required to identify new therapeutic targets. Over time, as more data are collected, there is great promise in the ability to better personalize patient care using this form of liquid biopsy, thereby steering through the challenging parts of relapsed or refractory cancer treatment.
Digging into the Challenges: Understanding the Tricky Parts of Modern Lung Cancer Treatment
While the prospect of more personalized medicine and biologically tailored approaches is exciting, it is also on edge with several challenging issues that need to be addressed. The integration of liquid biopsies into routine clinical practice, the redesign of radiotherapy protocols, and the timing of immunotherapy administration remain subjects of future research and debate.
The Challenge of Timing and Dosage
One of the more complicated pieces in the protocols for combining treatment modalities is the timing of when to administer immunotherapy. As evidenced by the PACIFIC-2 trial (NCT03519971) in stage III NSCLC, administering durvalumab (Imfinzi) concurrently with chemoradiotherapy only produced a marginal and statistically insignificant benefit compared to standard therapy alone. The absence of a significant improvement in progression-free survival underscores the need to carefully balance the dosage and administration timing to avoid unintended consequences, such as lymphodepletion.
Balancing Efficacy With Safety
The decision to integrate multiple treatment strategies also necessitates weighing the benefits against any potential risks. On one hand, enhancing tumor control can significantly extend survival; on the other hand, adding more treatments—especially those that are neurotoxic or immunologically suppressive—can introduce cognitive deficits and other severe side effects. Physicians must get around these pitfalls by collaborating closely with multidisciplinary teams that include oncologists, radiologists, and neurologists to define a treatment strategy that maximizes efficacy while mitigating harmful side effects.
Evaluating the Impact of Tumor Microenvironment on Treatment Effectiveness
The effectiveness of combined treatment approaches, such as immunotherapy with chemoradiation, is heavily influenced by the tumor microenvironment. The microenvironment’s composition, including the range and activity of immune cells present, plays a critical role in the therapeutic response. Understanding these subtle details can help to guide the timing and sequencing of treatments.
Immune Cell Dynamics During Treatment
Recent studies have indicated that the thoracic radiation used in chemoradiotherapy may reduce the number of available immune cells needed for a robust immunotherapy response. This depletion can lessen the overall impact of immunotherapy if used concurrently. Such findings imply that treatment regimens need to be rethought in order to preserve immune cell function. Instead of administering immunotherapy at the same time as radiation, delaying its initiation until after the completion of chemoradiotherapy might allow the patient’s immune system to recover and mount a more effective anti-tumor response.
Modulating the Tumor Microenvironment
Modifying the tumor microenvironment to encourage immune cell persistence and function is another promising area of research. Approaches such as using protective cytokines or exploring alternative dosing schedules could help mitigate the negative impact of radiation on the immune system. By finding effective ways to preserve these essential white blood cells during treatment, clinicians can potentially enhance the benefits of immunotherapy and improve overall survival outcomes.
Interdisciplinary Approaches: The Key to Better Outcomes
Modern oncology increasingly relies on interdisciplinary collaboration to address the many twists and turns of treatment strategies. Bringing together researchers from diverse fields—radiation oncology, medical oncology, pathology, and molecular biology—helps in piecing together the nitty-gritty details that may be hidden in traditional treatment plans.
Collaboration Between Laboratory and Clinical Teams
One of the most promising directions in cancer treatment comes from successful translational studies that bridge laboratory discoveries with clinical applications. The work at Indiana University is a prime example, where clinical observations are being paired with cutting-edge molecular studies. This integrated approach not only digs into the finer points of tumor biology but also helps identify new therapeutic targets and pathways that can be exploited in specific patient populations.
The Role of Multidisciplinary Conferences and CME Programs
Multidisciplinary conferences and continuing education programs play a super important role in ensuring that the latest findings from clinical trials are quickly translated into clinical practice. From updates on the latest FDA approvals to discussions of treatment sequencing in SCLC and NSCLC, these venues allow clinicians to share experiences and strategies. Such knowledge exchanges support decision-making in busy clinical settings, where each case requires careful deliberation over the small distinctions that define the best personalized care.
Patient-Centered Care in a Fast-Changing Field
While state-of-the-science therapies are advancing at a rapid pace, one of the major challenges remains ensuring that patients are supported and well-informed. The complexity of treatment choices can be overwhelming, and personalized care must be underpinned by clear communication about the risks and benefits of each approach.
Providing Clear Information About Treatment Options
Clinicians must find their way through communicating the subtle details of treatment regimens in an accessible manner. From explaining why immunotherapy may be delayed to discussing the potential side effects associated with PCI, it is essential that healthcare providers outline both the expected benefits and the potential drawbacks. Patient education sessions and support groups can play pivotal roles in ensuring that individuals feel prepared and confident in the decisions made regarding their care.
The Importance of Monitoring and Follow-Up
Given the nerve-racking nature of a cancer diagnosis, follow-up care becomes super important. Regular monitoring using advanced imaging techniques such as MRIs not only helps in detecting early signs of progression but also informs physicians about the ongoing health status of each patient. This strategy allows for timely adjustments in therapy, ensuring that any emerging resistance or side effects are managed appropriately. The government of care through close monitoring also helps maintain a high quality of life for patients, who can feel reassured that their treatment is being managed proactively.
Future Directions: Where Do We Go From Here?
As we continue to gather data from ongoing trials and translational research studies, many of the current approaches to lung cancer treatment will likely be refined. Future studies may offer more answers about the optimal sequencing of immunotherapy and chemoradiotherapy, the precise role of PCI in various stages of SCLC, and the best practices for using liquid biopsies to track tumor changes.
Potential Modifications in Treatment Protocols
Looking forward, the design of treatment protocols will probably hinge on a few key strategies:
- A greater emphasis on consolidative immunotherapy after chemoradiation to harness a more effective immune response.
- Refinement of PCI application based on individual patient risk profiles and the availability of regular high-resolution imaging.
- Expanded use of liquid biopsies to dynamically tailor treatments as the tumor evolves, ensuring that therapy remains personalized over time.
Maintaining Flexibility in Treatment Decisions
The ever-changing nature of cancer treatment demands flexibility. Clinicians will need to continuously update their approaches as new data becomes available, ensuring that every patient receives the most up-to-date care. This dynamic approach reinforces the idea that treatment is not a one-size-fits-all process but instead must be tailored to the individual nuances of each case.
The Role of Real-World Evidence in Shaping Future Standards
Real-world evidence (RWE) is emerging as a critical factor in informing treatment decisions. While controlled clinical trials provide a clear picture of efficacy and safety in an ideal environment, RWE offers valuable insights into how treatments perform in varied clinical settings. By integrating evidence from community practices, academic centers, and longitudinal studies, healthcare professionals can better understand the complicated pieces of treatment effectiveness in everyday practice.
Integrating Clinical Trial Data With Everyday Practice
Several studies, such as PACIFIC-2 and NRG-LU005, have highlighted both the promise and the potential pitfalls of adding immunotherapy to chemoradiotherapy. More importantly, these trials underscore the importance of not only relying on controlled data but also considering the outcomes observed in routine clinical settings. When combined, these insights allow practitioners to get into the absolute fine shades of personalized care, managing treatment based on the specific needs and responses of the patient population they serve.
Developing Guidelines That Reflect Real-World Challenges
Future guidelines should consider both clinical trial data and RWE to create recommendations that are both practical and scientifically robust. The challenge here is to create a balanced set of guidelines that address the slight differences observed between highly controlled environments and the unpredictable, dynamic situations encountered in everyday practice. Such an approach ensures that the recommendations remain useful and applicable to a broad range of patients.
Considering Quality of Life and Patient Outcomes
As new therapies and combinations become available, it is crucial to remember that improving overall survival should not come at the cost of quality of life. Issues such as cognitive decline, physical debilitation, and emotional strain can have long-lasting effects that are as significant as the disease progression itself. Therefore, treatment decisions must be informed by a holistic view of patient care.
Improving the Patient Experience
For many patients, the journey through cancer treatment is as much about managing side effects and maintaining a sense of normalcy as it is about fighting the disease. Physicians are increasingly called upon to offer support that addresses the whole person—emotionally, physically, and psychologically. This includes offering clear explanations about the benefits and possible burdens of treatments like PCI and consolidative immunotherapy, as well as providing access to counseling, nutritional guidance, and rehabilitation services.
Balancing Treatment Efficacy With Life Quality
When devising a treatment plan, caregivers must figure a path that prioritizes both the extension of life and the preservation of its quality. Some key considerations include:
- Carefully selecting patients for aggressive treatment based on their overall health and ability to tolerate potential side effects.
- Using advanced imaging and biomarkers to tailor treatment regimens to reduce unnecessary exposure to harmful side effects.
- Engaging in open dialogue with patients and their families to ensure that treatment decisions align with their personal values and lifestyle goals.
The Future is Personalized: Adopting a Patient-Specific Approach
The direction of modern oncology is unmistakably moving toward more precise, personalized care. With innovations like liquid biopsies and advanced imaging techniques, clinicians are now better equipped to track and adapt treatments in near real-time. This approach works through the small distinctions in tumor behavior and patient response to craft tailored treatment plans that offer the best chance for both survival and quality of life.
Utilizing Genetic and Molecular Data
In addition to relying on imaging studies, the analysis of genetic and molecular profiles is becoming a must-have component of modern cancer treatment. Genetic profiling can reveal key mutations and altered signaling pathways, and when combined with the dynamic data obtained from liquid biopsies, offers a comprehensive view of each tumor. This data can then guide clinicians in choosing targeted therapies, thereby avoiding some of the overwhelming side effects associated with a more generalized treatment approach.
Creating Personalized Treatment Roadmaps
Imagine each patient’s treatment as a journey, where cancer therapy is not one continuous path but rather a series of carefully planned stops and adjustments. By taking periodic blood tests, performing regular MRI scans, and tracking symptomatic changes, care teams can create flexible treatment roadmaps that adjust with each new piece of data. This patient-specific method is essential in managing the tangled issues of drug resistance and disease recurrence, ultimately helping improve outcomes over the long term.
Concluding Thoughts: Embracing the Complexity of Lung Cancer Treatment
In the final analysis, the modern approach to lung cancer treatment is a blend of art and science, where data-driven decisions meet the human desire for personalized care. The recent clinical trials challenge long-held assumptions about concurrent immunotherapy and chemoradiotherapy, while also encouraging a re-evaluation of established practices such as prophylactic cranial irradiation.
As we continue to gather new insights and address the confusing bits that remain in treatment guidelines, one thing is clear: personalized care is not simply an option; it is an essential evolution in the way we manage complex diseases like lung cancer. With ongoing studies and a commitment to iterative improvement, the future holds promise for treatment strategies that balance efficacy, safety, and quality of life for every patient.
While the twists and turns of modern oncology may seem intimidating at times, the steady march of research and the integration of advanced technologies hold the key to unlocking better outcomes. For clinicians, patients, and caregivers alike, it is a journey that demands persistence, continual learning, and above all, a patient-centered approach that treats each individual as unique. Through clear communication, interdisciplinary collaboration, and an unwavering commitment to evidence-based practice, the field of oncology is poised to make significant strides in the coming years.
The dialogue around lung cancer treatment is far from settled, with every new study adding more data to the conversation. As we figure a path forward, the importance of reviewing real-world evidence, embracing new personalized strategies such as liquid biopsies, and ensuring that quality of life remains at the forefront of all decisions cannot be overstated. In the end, it is this balanced approach—one that manages the intricate pieces of clinical complexity with a focus on the patient—that will define the future of lung cancer care.
In today’s rapidly evolving landscape of oncology, the onus is on us—clinicians, researchers, and patients—to get into the fine points and work through the most challenging aspects of treatment. By combining cutting-edge scientific advances with compassionate patient care, we can continue to push the boundaries of what is possible and steadily move towards a future where lung cancer becomes a manageable, and ultimately conquerable, disease.
As the journey continues, we must remain adaptable, continuously updating our methods to best serve our patients. The use of advanced imaging, coupled with dynamic treatment adaptations based on real-time biomarker data, is just one example of how we are starting to untangle the complicated pieces of modern cancer treatment. It is a time of cautious optimism, where every study, every trial, and every patient story contributes to a deeper understanding of this challenging disease.
In closing, the conversation surrounding the integration of immunotherapy, chemoradiation, and preventive interventions like PCI reminds us that progress in medicine is rarely linear. It is a process marked by trial and error, innovative thinking, and, most importantly, the relentless pursuit of better patient outcomes. With each step forward, we are learning to better navigate the tricky parts, balance efficacy with safety, and ultimately deliver care that is both scientifically sound and empathetically grounded.
This is an exciting time for oncology. With continuous improvements in our understanding and ever-increasing collaboration across disciplines, the promise of truly personalized, high-quality cancer care is more real than ever before. As we look ahead, the guiding principle remains clear: every patient deserves a treatment plan that is as unique as they are, one that respects the delicate balance between aggressive cancer treatment and the preservation of a life well-lived.
Originally Post From https://www.onclive.com/view/chemoradiotherapy-data-pci-controversy-and-translational-research-advance-sclc-care
Read more about this topic at
Small Cell Lung Cancer from Traditional to Innovative …
3 Things You Should Know About Evolving Strategies in …