Exploring APOE Deficiency and Its Anti-Tumour Impact in Liver Cancer
The latest research in liver cancer is opening up new possibilities in our fight against one of the world’s most challenging malignancies. Recent studies have brought attention to the role of APOE deficiency and how it can trigger anti-tumour activity through macrophages. In the complex, tangled issues of liver cancer treatment, scientists are beginning to piece together how targeting these immune cells might help steer through the maze of immunosuppression. This editorial takes a closer look at the promising research that explores the hidden complexities of the tumour microenvironment, the promising role of APOE+ tumour-associated macrophages, and the potential to use combination treatments to improve patient outcomes.
Understanding the Tumour Microenvironment in Liver Cancer
Liver cancer, notably hepatocellular carcinoma, is a disease loaded with problems. The tumour microenvironment is full of confusing bits, especially when it comes to the diverse population of cells that aid or hinder cancer progression. Among these cells, macrophages have emerged as key players in dictating how liver cancer grows and responds to therapies.
Macrophages in liver tumours exist in many forms, each carrying out different functions. Certain subsets can actually support the tumour by creating a protective niche against the body’s immune defenses. This situation can be intimidating for researchers who are trying to chart a clear path toward effective treatments. In contrast, other macrophage types might help the body fight off cancer cells if correctly targeted, as evidenced by the recent focus on APOE deficiency that appears to repurpose these cells into anti-tumour warriors.
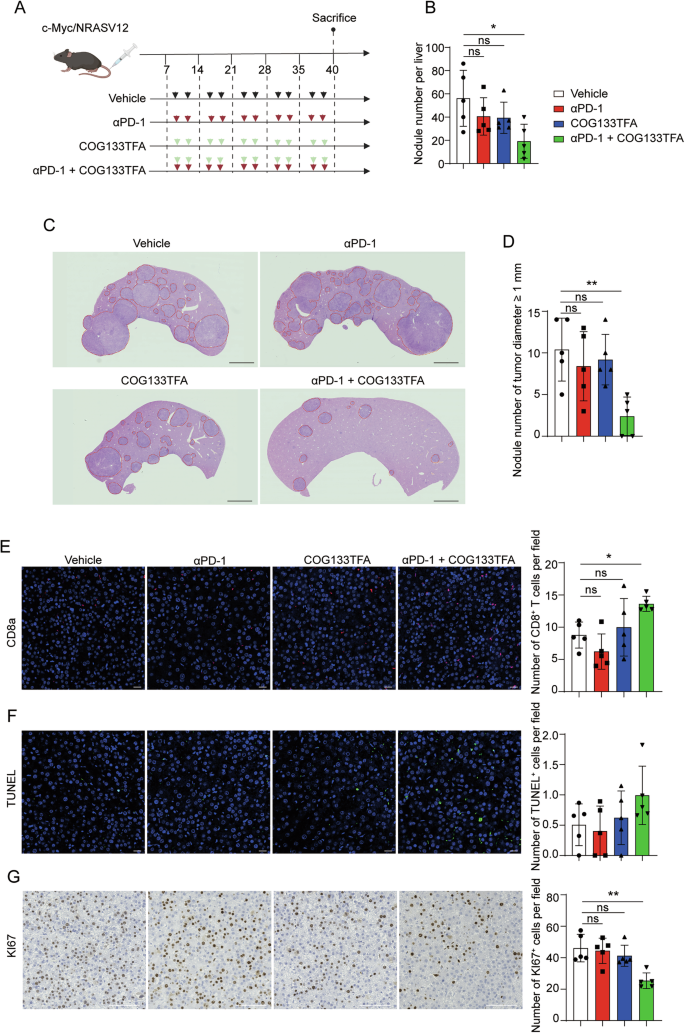
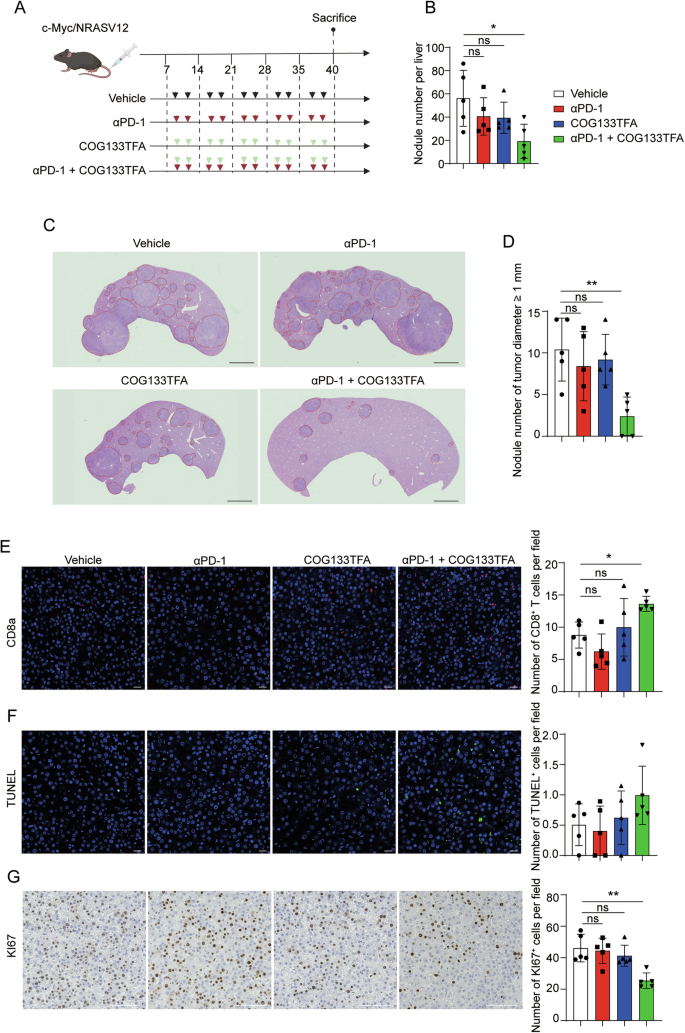
Apolipoprotein E (APOE) is usually known for its role in lipid metabolism, but its presence in tumour-associated macrophages (TAMs) introduces a challenging dynamic. Instead of merely being bystanders, these cells can contribute to the creation of a suppressive microenvironment. By expressing high levels of APOE, TAMs appear to interfere with the infiltration and function of CD8+ T cells, which are critical for anti-cancer immunity. The research highlights the need to figure a path that addresses these tricky parts of immune cell regulation.
Decoding the Tricky Parts of Targeting Tumour-Associated Macrophages
One of the biggest obstacles in liver cancer treatment is the inherent heterogeneity of macrophages. This heterogeneity means that when we poke around the tumour microenvironment, we find a range of different cells, each with its own set of subtle parts and fine shades influencing their behaviour. Some subsets, like the APOE+ TAMs, have been found to be more involved in dampening the immune response, creating a scenario in which the tumour can thrive.
Here are some of the key challenges in targeting these cells:
- Varied Cell Types: Not all macrophages are created equal. The differences among these cells require a tailored therapeutic approach rather than a one-size-fits-all strategy.
- Cholesterol Efflux and Immune Suppression: APOE+ TAMs are engaged in cholesterol efflux, a process that may inadvertently help to shroud the tumour in an immunosuppressive cloak. This bit of hidden complexity is critical when figuring out how to dismantle the tumour’s defences.
- Impact on T Cell Function: These cells seem to play a tricky role in hampering the infiltration of CD8+ T cells, which are essential for mounting an effective anti-tumour response. Overcoming this barrier is a key step toward restoring the body’s natural ability to fight the tumour.
Each of these problems, while daunting in its own right, provides a guidepost for researchers searching for smarter, targeted therapies. By reprogramming the role of these macrophages, we can potentially turn their suppressive functions into mechanisms that empower the immune response.
Overcoming Immunotherapy Resistance Through APOE Blockade
While immunotherapy has shown remarkable promise in many cancers, its success in liver cancer has been limited, often because of an immunosuppressive milieu controlled by cells such as APOE+ TAMs. Many patients, unfortunately, show resistance to immune checkpoint blockade (ICB), a process that seemingly reflects the influence of these problematic macrophages. In tumours that are non-responsive to ICB, the heightened intensity of APOE+ macrophages is a notable hallmark.
Recent findings suggest that limiting APOE activity in these cells can remove one of the suppressive layers that shield the tumour. For example, studies in immunotherapy-resistant mouse models reveal that when the APOE pathway is inhibited, there is a pronounced increase in CD8+ T cell infiltration. This finding is critical because it opens up the possibility of combination therapies: using APOE inhibitors alongside standard ICB can lead to more dramatic tumour control.
Working through the tangled issues of immunotherapy resistance involves:
- Synergistic Effects: The combination of APOE blockade with anti-PD-1 therapies appears to be more effective than either strategy alone.
- Modulation of Immune Cell Activity: Reducing APOE levels reprograms macrophage function. This change boosts the ability of CD8+ T cells to target and kill tumour cells.
- Potential for Broader Applications: Although primarily studied in liver cancer, these findings could have implications for other cancers that similarly employ immunosuppressive strategies.
Ultimately, these insights empower us to start reimagining how we design therapies that are smart enough to tackle not only the tumour cells themselves but also the supportive yet invasive environment that helps these tumours grow.
Improving CD8+ T Cell Infiltration and Function in Tumour Microenvironments
The significance of CD8+ T cells in cancer defense is a common theme in oncology discussions. These cells are the body’s natural foot soldiers in fighting off malignancies. However, in many liver tumours, the immune system’s response is dulled by the actions of suppressive macrophages. Consequently, a core focus for many researchers is on the treatment strategies that can support and enhance the infiltration and functioning of these T cells.
A key observation in recent studies is that higher levels of APOE in tumour-associated macrophages correlate with reduced CD8+ T cell presence in the tumour microenvironment. This inverse relationship offers the possibility that by curbing the action of APOE+ TAMs, one might restore CD8+ T cell activity, thereby increasing the efficacy of treatments like checkpoint inhibitors.
Some of the strategies being considered include:
- APOE Inhibitors: Drugs designed to block APOE can potentially reduce the suppressive effects of macrophages, thereby allowing more CD8+ T cells to enter the tumour region.
- Combination Therapy: Using a combined approach where APOE inhibitors are paired with well-known immunotherapies can boost the overall anti-tumour response.
- Meta-Analysis of Immune Profiles: Looking at patterns across different patients can help in identifying who might benefit most from these types of interventions. This involves digging into the fine points of patient immune profiles and fine-tuning therapies accordingly.
By boosting the levels and functionality of CD8+ T cells, clinicians can potentially shift the balance in favor of tumour regression. It is a delicate balancing act: on one side, we have the tumour’s ability to create a nerve-racking environment through suppressive signals, and on the other, the body’s own defenses that, if properly rallied, could overcome these challenges.
Clinical Perspectives: Rethinking Treatment for Hepatocellular Carcinoma
From a clinical viewpoint, the research into APOE deficiency and TAMs carries significant promise. The discovery that APOE levels in macrophages can strongly influence the success of immunotherapies presents a clear, actionable target for future treatments. With liver cancer patients often facing an overwhelming burden of disease, this research provides a ray of hope for more targeted, effective interventions.
Prior clinical trials have provided a mixed bag of results when it comes to checkpoint inhibitors in liver cancer. However, the introduction of APOE blockade could reshape the landscape of immunotherapy. For example, patients with high APOE+ TAM intensity are often linked with poorer overall survival and lower CD8+ T cell infiltration. Hence, by directly targeting these cells, we might see not only improved tumour control but also enhanced overall survival rates.
Key clinical considerations include:
- Predictive Biomarkers: Determining APOE expression levels can serve as an essential indicator to decide which patients might respond best to combination immunotherapy strategies.
- Patient Stratification: By sorting out patients based on the extent of APOE+ macrophage infiltration, clinicians can tailor treatments to individual profiles, enhancing the precision of care.
- Combination Regimens: Given the evidence from experimental models, it is crucial to explore therapy regimens that combine APOE blockade with immune checkpoint inhibitors to achieve synergistic benefits.
The clinical trials planned for the future should closely examine these aspects, making sure that the fine details of each patient’s tumour biology are taken into account. This approach is key to designing smarter therapies that can outmaneuver the tumour’s natural defenses and overcome the many tricky parts associated with liver cancer treatment.
Insights into Cholesterol Metabolism and Immunosuppression
An unexpected twist in the story of liver cancer treatment is the connection between cholesterol metabolism and immune function. APOE is a central molecule in cholesterol transport, and its overexpression in tumour-associated macrophages appears to tie cholesterol efflux to immunosuppression. When macrophages engage in cholesterol efflux, they not only alter their own metabolism but also shape the surrounding environment to favour tumour growth.
This relationship is filled with subtle details that are critical to understanding the broader picture of immunotherapy resistance. On one hand, modulating cholesterol levels in the tumour microenvironment might be a promising method of reprogramming the immune cells. On the other, the precise regulation of cholesterol metabolism in these cells is laden with challenging bits that are not yet fully understood.
Recent preclinical studies have shown that manipulating the cholesterol pathway in macrophages can help reverse the suppressive effects on CD8+ T cells. The cholesterol efflux process, when tamped down through APOE deficiency, appears to rewire the macrophages into cells that are less friendly to the tumour and more likely to support anti-tumour responses.
This area of research highlights several exciting opportunities:
- Metabolic Reprogramming: By targeting the metabolic pathways linked to cholesterol metabolism, clinicians may be able to diminish the immunosuppressive behaviour of TAMs.
- Combination with Statins: There is speculation around the potential benefits of combining APOE blockade with statins, drugs known for their cholesterol-lowering effects, in order to further dilute the suppressive microenvironment.
- Immune-Metabolic Crosstalk: Understanding the dialogue between metabolic changes and immune regulation could pave the way to novel treatments that address both the energetic and immunologic demands of the tumour microenvironment.
By clarifying the fine points of this crosstalk, research not only enhances our basic understanding of liver cancer biology but also points toward new venues for therapeutic intervention. Every small twist in this process is a potential key to unlocking more effective treatments.
Strategies for Combination Therapy in Hepatocellular Carcinoma
The last decade has seen a significant shift in cancer treatment strategies, with combination therapy emerging as a promising approach. In the case of hepatocellular carcinoma, the concept of combining APOE inhibitors with standard immune checkpoint inhibitors represents a powerful strategy to tip the balance in favour of the patient’s own immune system.
Combination strategies are designed to tackle multiple fronts at once. In liver cancer, while immunotherapies such as anti-PD-1 have shown some success, their effectiveness is often hampered by the presence of immunosuppressive macrophages. By introducing an APOE inhibitor into the regimen, the immunotherapy is given a critical assist that may help lift the barrier against CD8+ T cell activation and recruitment.
The current model for combination therapy can be visualized with the following steps:
| Step | Action | Expected Outcome |
|---|---|---|
| 1 | Administer APOE Inhibitor | Reduce immunosuppressive signalling from TAMs |
| 2 | Apply Anti-PD-1 Therapy | Enhance CD8+ T cell activation and tumour infiltration |
| 3 | Monitor Immune Response | Assess tumour shrinkage and immune reprogramming |
| 4 | Adjust and Tailor Therapy | Optimize therapy based on individual patient data |
This table outlines a suggested framework that researchers and clinicians can use to investigate the possibility of a multi-pronged approach. Each step involves strategic decisions that take into account the small distinctions in patient immune profiles and the more complicated pieces of the tumour biology puzzle.
Optimizing Patient Outcomes Through Personalized Therapy
One exciting aspect of this research is the potential to tailor treatments to individual patients based on specific biomarkers, such as the level of APOE expression on tumour-associated macrophages. This personalized approach is becoming increasingly important in today’s era of precision medicine.
By assessing a patient’s immune profile and tumour biology, clinicians can:
- Stratify Patients Effectively: Group patients based on unique characteristics, such as the intensity of APOE+ TAMs and the extent of CD8+ T cell suppression.
- Adjust Doses and Timings: Fine-tune the regimen depending on how a patient’s immune system responds to both the immunotherapy and the APOE blockade.
- Increase the Odds of Success: Ensure that each patient receives a custom-tailored treatment plan that maximizes the chances of overcoming the tumour’s nerve-racking defenses.
These strategies highlight the moves towards a more individualized approach, where every patient is treated not as a statistic but as a complex individual with nuanced needs. It is a shift that increasingly acknowledges the importance of recognizing and working through the little details that determine treatment success.
Challenges and Future Directions in Research
Although the recent findings on APOE deficiency in liver cancer are inspiring, there remain plenty of tricky parts and tangled issues that researchers need to sort out before these insights are translated into widespread clinical use. Some of the key challenges include:
- Understanding Macrophage Heterogeneity: There is still much to learn about the distinct subsets of TAMs beyond the APOE+ group. More research is needed to picture the full array of these cells and how they interact with the tumour and each other.
- Long-Term Effects of APOE Blockade: While early studies in mouse models are promising, it remains to be seen how long-term inhibition of APOE will impact overall metabolism and immune regulation in humans.
- Optimizing Combination Regimens: Figuring a path to combine various therapies in the most effective way is an ongoing challenge. Determining the right dosages, treatment intervals, and patient selection criteria is a nerve-racking process that requires extensive clinical trials.
- Managing Side Effects: As with any targeted therapy, there is a potential for off-target effects or other unexpected issues. Close monitoring and adjustments will be critical as these therapies move from the lab to the clinic.
Despite these issues, the potential advantages of using APOE inhibitors to enhance immunotherapy are undeniable. By taking a closer look at these challenging bits of liver cancer biology, the medical community can work towards developing smarter, more refined treatment protocols that not only extend survival but also improve the quality of life for patients.
Reflections on the Future of Liver Cancer Treatment
As we poke around in the evolving landscape of liver cancer research, the convergence of immunotherapy and cellular metabolism offers a promising horizon. The research on APOE deficiency underscores that sometimes the key to overcoming a disease lies in targeting not just the cancer cells, but also the broader ecosystem in which they thrive. The emerging data reinforces the concept that immune cells, particularly TAMs, play a critical role in dictating the fate of the tumour by either hampering or helping the immune response.
This body of work is a reminder that in the fight against cancer, no detail should be considered too small. Whether it’s the fine points of cholesterol transport, the subtle parts involved in T cell activation, or the little twists in macrophage function, every component has a role in either promoting or fighting the disease. Researchers are now better equipped to figure a path through this tangled web by targeting multiple angles concurrently, which is a must-have strategy in modern oncology.
The integration of APOE inhibition with existing immunotherapeutic approaches could transform the management of hepatocellular carcinoma. By dismantling the protective barrier imposed by APOE+ TAMs, the door opens for T cells to regain their tumour-fighting capabilities. With more studies aimed at clarifying these fine shades of immune regulation, the future holds the promise of more effective, personalized treatments that acknowledge the nerve-racking twists and turns of cancer biology.
Looking Beyond the Laboratory
While the current research is predominantly based on laboratory and animal studies, it paves the way for future clinical trials. The next steps involve taking these early promising results and testing them in a broader, more diverse patient population. This phase is crucial in determining whether the observed benefits in controlled settings can translate to real-world improvements.
This phase of research will involve:
- Multicentric Clinical Trials: By involving multiple research centers and diverse patient groups, these studies can validate the effectiveness of APOE blockade across various demographic and genetic backgrounds.
- Monitoring Long-Term Outcomes: Extended follow-up periods will help in assessing not only tumour regression but also the overall survival and quality of life improvements.
- Adjusting Therapeutic Protocols: Ongoing monitoring and adaptation of the treatment regimens will be necessary as new insights emerge regarding the subtle details of immune regulation and metabolic processes.
In the end, the translation of these findings into clinical practice represents a significant step forward in the overall strategy for liver cancer treatment. It is a journey that will require coordination between laboratory scientists, clinicians, and pharmaceutical developers, each working through the various layers of this complex challenge.
Embracing a Multi-Disciplinary Approach
One clear takeaway from the ongoing research is that a multi-disciplinary approach is essential. On one hand, there is the need for a deep understanding of cellular metabolism and the metabolic twists that impact immune cell functions. On the other, there is an equally pressing need to appreciate the minute details of immunology that govern T cell behaviour and macrophage responses.
Integrating these perspectives requires collaboration between experts in modern medicine, alternative medicine, nutritional science, and even fields like bioinformatics and systems biology. Together, these areas of expertise can help piece together the various parameters that influence successful treatment. For instance:
- Immune Profiling: Advanced technologies like single-cell sequencing provide clarity on the subtypes of macrophages within tumours. Understanding these profiles will help in developing targeted approaches.
- Metabolic Analyses: Research into cholesterol metabolism and its impact on the immune system can yield invaluable clues about how to create a less supportive environment for the tumour.
- Nutritional Interventions: While still in the early stages, combining nutritional strategies with pharmaceutical interventions may further tip the balance in the body’s favour.
These collaborative efforts underscore the necessity of working together and sharing expertise. The challenge is indeed on edge, but by managing your way through these interconnected disciplines, we can arrive at treatments that are both effective and sustainable.
Conclusion: A New Horizon in Liver Cancer Treatment
The journey towards more effective liver cancer treatments is full of confusing bits and challenging twists. However, the research on APOE deficiency offers a ray of hope in an area that has long been overwhelmed by the protective tactics of the tumour microenvironment. By targeting the specific subset of tumour-associated macrophages that express high levels of APOE, there is a pathway emerging to remove the shield that prevents CD8+ T cells from doing their job.
While much of the work is still in its early stages, the potential for combining APOE blockade with established immunotherapies is exciting. Such a dual approach addresses both the inflammatory mediators and the metabolic factors that support the tumour’s growth. In doing so, it not only works through the confusing bits of tumour biology but also unveils new paths that promise more refined, personalized treatments.
This opinion piece reflects on the exciting progress made in recent studies and celebrates the promise of future advances. It is a call to continue investigating the fine points of immune cell regulation, to not be daunted by the nerve-racking challenges ahead, and to remain optimistic about the prospects of turning the tide against liver cancer.
In an era when precision medicine is increasingly important, the integration of APOE inhibitors as a component of combination therapy stands out as a critical, perhaps even game-changing, strategy. By utilizing smart approaches to stimulate the immune system and reprogram harmful macrophages, we can hope to see improved overall survival rates and better quality of life for patients suffering from this aggressive disease.
Thus, while the journey is still long and the challenges remain plenty, the future of liver cancer treatment looks promising. As researchers continue to dig into and unravel more of the complex interplay between immune cells and metabolic processes, there is every reason to believe that more effective, patient-specific therapies are on the horizon. It’s a time of cautious optimism—a time when the efforts to piece together the little details of cancer’s hide-and-seek game may finally pay off.
Ultimately, the evolving story of APOE deficiency and its anti-tumour activity in liver cancer is a vivid example of how modern research can turn seemingly overwhelming problems into promising therapeutic opportunities. By piecing together these essential insights, we are not only advancing our understanding of cancer biology but also laying the groundwork for treatment paradigms that could change the lives of countless patients.
Originally Post From https://www.nature.com/articles/s41417-025-00936-2
Read more about this topic at
Orchestrating Macrophage activation and elevating the …
Macrophage-Targeted Therapy Unlocks Antitumoral Cross …