Bria-IMT in Metastatic Breast Cancer: A Promising Frontier in Clinical Innovation
This editorial examines the latest phase 2 trial findings for Bria-IMT therapy in heavily pretreated metastatic breast cancer populations. Recent data suggest that combining Bria-IMT with checkpoint inhibitors could offer enhanced overall survival benefits compared to current standard-of-care regimens. In this discussion, we will take a closer look at the trial’s findings, the design features of the study, and what these results might mean for future treatment options. We will also explore the patient journey, safety aspects, and the broader context of evolving immunotherapy strategies.
Recent clinical trials and expert opinions have generated significant interest in integrating novel immunotherapy approaches for late-stage metastatic breast cancer. One such approach, pioneered by BriaCell Therapeutics, is the Bria-IMT regimen, which is being studied in combination with checkpoint inhibitors. The early phase 2 data indicate a survival rate at one year reaching 52% among a group of heavily pretreated patients, many of whom had already progressed through multiple lines of therapy.
Exploring Combination Therapy: Bridging Bria-IMT and Checkpoint Inhibitors
The study under review recruited patients with metastatic breast cancer who had previously undergone numerous treatments, including checkpoint inhibitors and antibody-drug conjugates. This subgroup, traditionally known for having a nerve-racking treatment history due to limited alternatives, showed promising outcomes when administered the combination therapy.
While many experts agree that the management of such heavily pretreated patients can be intimidating, the survival data from the trial offers a ray of hope. With a reported 52% of patients surpassing the one-year survival milestone, the combination of Bria-IMT with checkpoint inhibition appears to offer a path through the tangled issues that hinder traditional monotherapy results.
In many ways, this study reflects a careful rethinking of the cancer treatment journey. Instead of simply relying on established regimens, researchers are now working through new combination strategies. By integrating Bria-IMT with immunotherapeutic agents, they intend to change the narrative from an almost off-putting cycle of failed treatments to one brimming with cautious optimism and improved survival outcomes.
Heavily Pretreated Patients: A Closer Look at Survival Gains
One of the remarkable aspects of the trial is that it focused on a patient population with a median history of six prior lines of therapy. The survival benefits noted—particularly with two patients demonstrating overall survival extending to over 30 and 38 months—are significant. These outcomes suggest that, despite the intimidating past treatment failures, there may be a new way to find one’s way through advanced metastatic breast cancer.
For many patients who are faced with progressively worsening conditions, the survival data represent not only extended life but also a chance at improved quality of life. The study’s findings also underline the importance of looking at survival outcomes beyond the immediate treatment period. As the final median overall survival (OS) calculations are awaited, the fact that some patient subgroups are still alive at the time of data cutoff further reinforces the potential of this regimen.
This combination therapy might be an example of a breakthrough for patients who are loaded with issues arising from previous treatment failures. In addition, these data give both patients and clinicians the reason to bear the hope that there is a way through the confusing bits and twists and turns of metastatic breast cancer treatment strategies.
Understanding the Treatment Regimen: A Detailed Breakdown
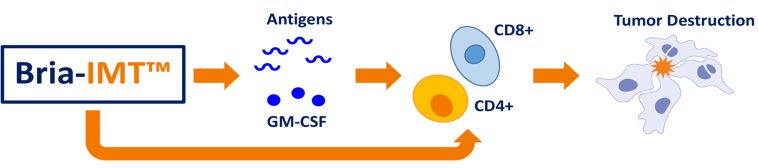
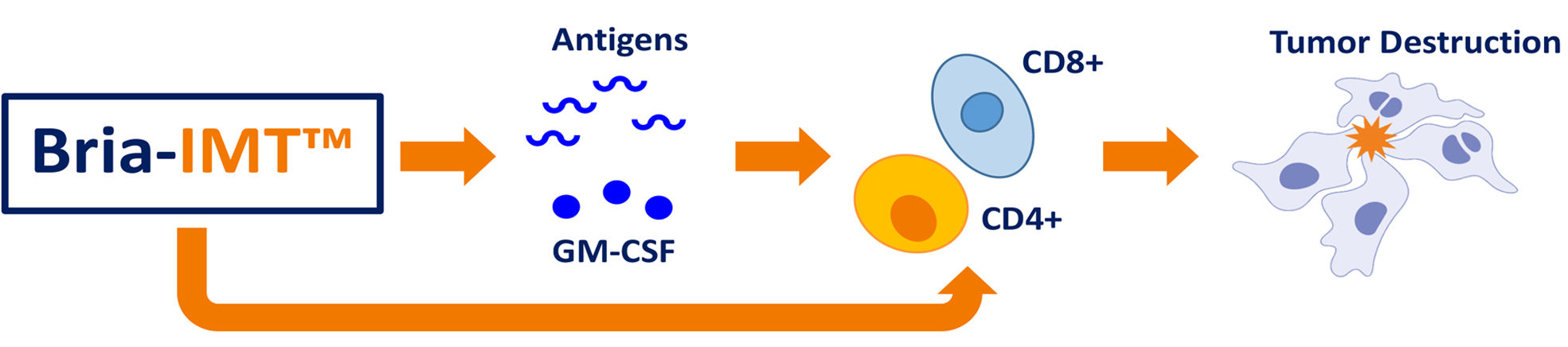
The Bria-IMT regimen is sophisticated in its design. In the study, the treatment was administered in 3-week cycles and combined several modalities: a low dose of cyclophosphamide, an intradermal vaccine component (SV-BR-1-GM), along with checkpoint inhibitor infusions and intradermal interferon. The coordination of these therapeutic pieces is designed to not only stimulate an immune response but also fine-tune the body’s ability to fight the cancer cells effectively.
Below, we offer a simplified table summarizing the regimen components:
| Component | Description | Administration Details |
|---|---|---|
| Cyclophosphamide | Chemotherapy agent used to suppress regulatory immune cells | 300 mg/m² on days –2 and –3 |
| SV-BR-1-GM Vaccine | Intradermal vaccine to stimulate immune response | Divided into 4 inoculations starting on day 0 |
| Checkpoint Inhibitor | Immunotherapy agent to release brakes on T-cells | Given via infusion during treatment cycle days 1 to 3 |
| Interferon | Immunomodulatory agent to enhance the local immune reaction | Administered intradermally alongside the vaccine |
This structured regimen reflects careful attention to the little details that can make a meaningful difference in treatment outcomes. By carefully calibrating both the dosage and scheduling, the study’s organizers are essentially trying to find a sweet spot where the patient’s immune system is stimulated without being overwhelmed by the toxic effects usually associated with chemotherapy and other immunomodulators.
Phase 2 Findings: The Emerging Survival Signal
The phase 2 segment of the trial aimed to evaluate the combination strategy’s efficacy in increasing overall survival, particularly among patients who did not benefit from previous therapies. Although the final median overall survival data is pending, interim results have been encouraging. The promising 52% one-year survival benchmark is a notable improvement compared to survival expectations from existing standard-of-care regimens.
It’s important to note that the trial enrolled 54 patients in total, a small but significant group. Of these, 25 patients exhibited the noteworthy survival benefit, and ongoing results indicate that this benefit might extend further as more patient data become available. The fact that no patients discontinued treatment due to Bria-IMT-related adverse effects is significant; this suggests that the regimen is well-tolerated even when dealing with those with advanced disease and multiple prior treatments.
Experts, including Dr. Adam M. Brufsky from the University of Pittsburgh School of Medicine, have expressed cautious optimism based on these results. According to him, the phase 2 data indicate a robust survival signal paired with a tolerable safety profile—a combination that could potentially shift treatment paradigms for patients at this critical stage of disease progression.
Study Design and Future Directions: Planning the Phase 3 Trial
The phase 2 study has set the stage for a more extensive phase 3 trial, which will provide further clarity on the survival benefits and broader safety profile of the Bria-IMT regimen. The upcoming trial is designed as a randomized study, and participants will be divided into three groups: one receiving the Bria-IMT regimen alongside combination therapy, another receiving an active comparator regimen, and a third group receiving the Bria-IMT regimen alone.
This approach allows researchers to assess not only the efficacy of the combination therapy but also to pinpoint which elements contribute most significantly to the survival benefits. The trial’s primary endpoint is overall survival, while secondary endpoints include progression-free survival, response rates, clinical benefit rate, as well as assessments of quality of life and central nervous system event-free survival. Such comprehensive endpoints are critical to truly understand the fine points of treatment effectiveness.
For patients eligible for the study, the inclusion criteria are quite stringent. Eligible participants must be 18 years or older, have a histologically confirmed diagnosis of locally recurrent unresectable and/or metastatic breast cancer, and have experienced disease progression despite prior therapy. Additionally, participants must have no meaningful alternative therapy available and an expected survival of at least four months. Performance scores (ECOG 0 to 2) further stratify which patients can safely participate in the trial.
This rigorous screening process assures that the study’s findings will be applicable to the real-world patient population, especially those who have already navigated the tricky parts of multiple treatment failures. With the enrollment of initial patients, the researchers plan to use the data to steer through the uncertain waters of metastatic breast cancer management, paving the way for future treatment optimization.
Checkpoint Inhibitors: An Essential Building Block of Modern Oncology
Checkpoint inhibitors have revolutionized cancer treatment by significantly improving outcomes in several malignancies. Their role, particularly in the treatment of metastatic breast cancer, is to help the immune system recognize and eliminate cancer cells. In the context of the phase 2 study, the addition of these agents to the Bria-IMT regimen is thought to boost the body’s natural anticancer mechanisms.
The combination of a vaccine-like therapy with checkpoint inhibition is designed to provide two key benefits. First, the vaccine component stimulates the immune system directly against tumor antigens. Second, the checkpoint inhibitor removes the brakes that often hinder an effective immune response in cancer patients, allowing the activated T-cells to more efficiently target malignant cells.
This dual action represents a fresh approach in a field where treatment options can sometimes feel limiting. For patients whose cancer has not responded to traditional methods, this strategy offers a novel method to dig into the problem and potentially turn around outcomes that were once considered off-putting or even impossible.
Safety and Tolerability: Addressing the Concern for Late-Stage Patients
In any oncologic treatment, particularly one where patients have already endured multiple rounds of therapy, the safety profile of a new regimen is critical. In this phase 2 trial, no treatment discontinuations were reported as a direct result of Bria-IMT administration. This finding suggests that the regimen is not only effective but also well-tolerated even in populations riddled with health challenges.
Patients who have faced the nerve-racking side effects of numerous prior treatments can be reassured by the fact that the Bria-IMT regimen appears to have a manageable side effect profile. Clinicians and researchers alike agree that while efficacy in extending survival is of paramount importance, ensuring that patients maintain a reasonable quality of life is equally critical. The observed tolerability is one of the promising fine shades in these early results.
The study design carefully monitors adverse events, and the early data has not flagged any safety concerns that would discourage further investigation into the combination treatment. This safety net is crucial to building confidence among both practitioners and patients when considering new treatment approaches for advanced stages of disease.
Expert Opinions: Bridging Data With Clinical Practice
Leaders in the field of medical oncology have provided thoughtful commentary on these early findings. Dr. Adam M. Brufsky underscored the potential of the Bria-IMT regimen, stating that the phase 2 data not only highlight a robust survival signal but also provide reassurance regarding the regimen’s safety. Similarly, Dr. Aditya Bardia from UCLA has noted that many patients progress despite available therapies, and new approaches like Bria-IMT represent hopeful alternatives that could change the current treatment landscape.
These expert opinions are invaluable. They help to guide the clinical community through the slight differences between traditional treatment regimens and emerging combination therapies. By integrating insights from both clinical efficacy and patient tolerability, oncologists are better equipped to find their way through the complicated pieces of modern metastatic breast cancer treatment.
Furthermore, the international research community is watching closely. The upcoming phase 3 trial, with its more detailed and randomized study design, promises to further clarify the role of Bria-IMT combination therapy in clinical practice.
Patient Perspectives: Hope Amid Trials and Turbulence
Patients with metastatic breast cancer often describe their journey as a path filled with tangled issues and overwhelming challenges. The introduction of new therapies like Bria-IMT not only provides a potential survival boost but can also reinvigorate the hope necessary to push through difficult times. Patients and advocates alike have expressed optimism that new combination treatments can offer more than just clinical benefit—they might also improve day-to-day quality of life.
It is essential for healthcare providers to communicate this balanced message: while the journey through advanced cancer treatment is full of twists and turns, emerging data from trials like this one offer a promising avenue to explore. With ongoing research and phase 3 studies on the horizon, patients have an opportunity to be part of a future where novel immunotherapy strategies help to steer through the confusing bits of repeated treatment failures.
As new therapies become available, shared decision-making between clinicians and patients becomes ever more critical. The use of patient-friendly language to explain treatment options—while addressing both the potential benefits and risks—can help patients feel empowered. After all, having a clear understanding of treatment rationale and expected outcomes is key to navigating not just the disease itself, but also the overall treatment experience.
Integrating Research With Real-World Practice: A Multifaceted Challenge
The translation of clinical trial results into everyday practice does not happen overnight. Healthcare professionals must find a path between the controlled environment of clinical studies and the unpredictable nature of routine care. This process involves addressing a variety of challenges, including the need to manage the tricky parts of dosing, scheduling, and patient selection, as well as ensuring that the potential benefits can be delivered safely.
In the case of the Bria-IMT regimen, the ongoing phase 3 studies will be crucial in providing additional data that can help clarify these fine shades of treatment application. The study’s design, which includes randomization into multiple treatment arms and detailed monitoring of various endpoints, reflects a meticulous approach that bridges the gap between research and practice.
For clinicians, the challenge is to manage patient expectations while waiting for definitive phase 3 data. It can be nerve-racking to balance optimism with caution, particularly when early results hint at an improved survival signal. Patient-centered care remains paramount, and this means that healthcare teams must prepare to discuss these novel therapies in a clear, balanced manner.
A bullet list highlighting the steps to bridge research and clinical application might include:
- Review and understand detailed clinical trial data.
- Educate patients about both known benefits and potential risks.
- Monitor emerging data from phase 3 trials and post-market studies.
- Adjust treatment protocols based on real-world observations.
- Maintain open communication channels with research teams for ongoing updates.
The integration of emerging therapies into everyday practice is as much about managing the science as it is about managing perceptions. Clinicians must figure a path through both the clinical data and the patient experience to ultimately improve outcomes for those with advanced metastatic breast cancer.
Future Directions: What Lies Ahead for Bria-IMT and Metastatic Breast Cancer Treatment?
The early promise of Bria-IMT combination therapy in the phase 2 trial breathes new life into the ongoing search for better treatments for metastatic breast cancer. As the data matures and additional patient outcomes are analyzed, there is every reason to believe that this approach could become a super important component of future treatment regimens.
Looking ahead, the transition into phase 3 will be the next critical step. The expanded trial will allow researchers to piece together even more of the subtle parts that contribute to survival and quality-of-life outcomes. Additionally, this larger study will help clarify which patient subgroups benefit the most from combination therapy, offering practitioners clearer guidelines on patient selection.
Some potential future research directions include:
- Comparative analysis of Bria-IMT vs. other emerging immunotherapy regimens.
- Investigation into biomarkers that predict treatment response to combination therapies.
- Long-term studies assessing the quality of life and functional status of survivors.
- Expanded trials focusing on different metastatic breast cancer subtypes.
- Real-world evidence studies that corroborate clinical trial findings.
An integrated research agenda that includes these directions will be essential for developing a comprehensive treatment framework. By continuously monitoring the outcomes, refining treatment protocols, and engaging in multi-center collaborations, the healthcare community can dig into these research questions and answer the pressing challenges of late-stage metastatic breast cancer care.
Weighing the Benefits and Challenges: A Balanced Opinion
When reviewing the early evidence, it is crucial to keep in mind that while the data is promising, more work remains. The complex pieces of cancer therapy require nuanced interpretation, and clinicians must stay abreast of ongoing developments. On the one hand, the survival benefit observed in a population that has already navigated a maze of treatments gives cause for celebration. On the other hand, the trial’s relatively small sample size, the pending median overall survival calculation, and the challenges entrenched in the study design necessitate a careful, measured approach.
This editorial encourages healthcare providers to remain cautiously optimistic. While the trial data offers hope and potential direction, it also underscores the ongoing need for rigorous clinical validation. Ultimately, when new treatment options emerge, they must be evaluated not only on the basis of survival statistics but also on the holistic impact they have on a patient’s life.
In practice, this means that oncologists need to work through the slight differences between emerging and established therapies. They should have comprehensive discussions with patients about both the promising outcomes and the possible risks. The ultimate goal is to pave a smoother treatment journey—one where patients feel both empowered and supported in their fight against metastatic breast cancer.
Conclusion: Paving a New Course in Metastatic Breast Cancer Care
The evolving landscape of metastatic breast cancer treatment, as highlighted by the phase 2 trial for Bria-IMT, opens up exciting possibilities. The combination therapy of Bria-IMT with checkpoint inhibitors offers a potential path through the intimidating challenges faced by patients who have exhausted multiple treatment lines. Early data suggest that this approach not only offers extended survival but does so with a tolerable safety profile, addressing some of the nerve-racking concerns often associated with advanced cancer care.
As we look toward the phase 3 trial and further studies, it is essential for clinicians, researchers, and patients to work together. This collaboration will ensure that data from pioneering studies are translated into effective, everyday treatments that can genuinely make a difference in patient outcomes. Managing your way through the tricky parts of metastatic breast cancer treatment requires not only cutting-edge science but also compassionate care and clear communication.
In closing, the promise seen in the Bria-IMT trial is a reminder that even in the face of daunting treatment histories, new modalities can shine a light on fresh therapeutic pathways. While no single treatment is a magic bullet, this innovative regimen adds another key component to the multifaceted approach needed to tackle metastatic breast cancer. As research continues, it will be crucial to keep a balanced view—celebrating the survival benefits while remaining vigilant about managing any potential risks. With further validation and collaboration, Bria-IMT might well become a super important tool that reshapes our understanding and management of advanced breast cancer.
In summary, the early success of the Bria-IMT regimen underscores the importance of continuously re-examining and refining cancer therapy strategies. By embracing a multi-modal treatment approach, integrating combination therapies, and remaining open to new clinical data, we can better support patients on their challenging journey. The twists and turns of metastatic breast cancer treatment may be complicated and intimidating, but with dedication to innovation and patient-centered care, there is renewed hope for improved outcomes and quality of life.
Originally Post From https://www.cancernetwork.com/view/bria-imt-yields-updated-survival-benefit-in-metastatic-breast-cancer
Read more about this topic at
Sturdy Outdoor Survival Mirror Hiking Camping Boating …
Best Signal Mirror for Rescue and Survival