Cell Therapy Revolution: Broadening Access Beyond Major Cancer Centers
The field of cellular therapy is undergoing a remarkable evolution, promising groundbreaking treatments for patients battling cancer. As emerging therapies such as autologous tumor-infiltrating lymphocyte (TIL) cell therapy and engineered T cell receptor (TCR)-T cells show significant potential, experts are increasingly optimistic about their future deployment outside traditional academic centers. In this editorial, we take a closer look at the future of specialized cell therapies, the challenges inherent in expanding access to community clinics, and how these innovative treatments might soon be available to a broader patient base.
Embracing Specialized Cell Therapies in Modern Oncology
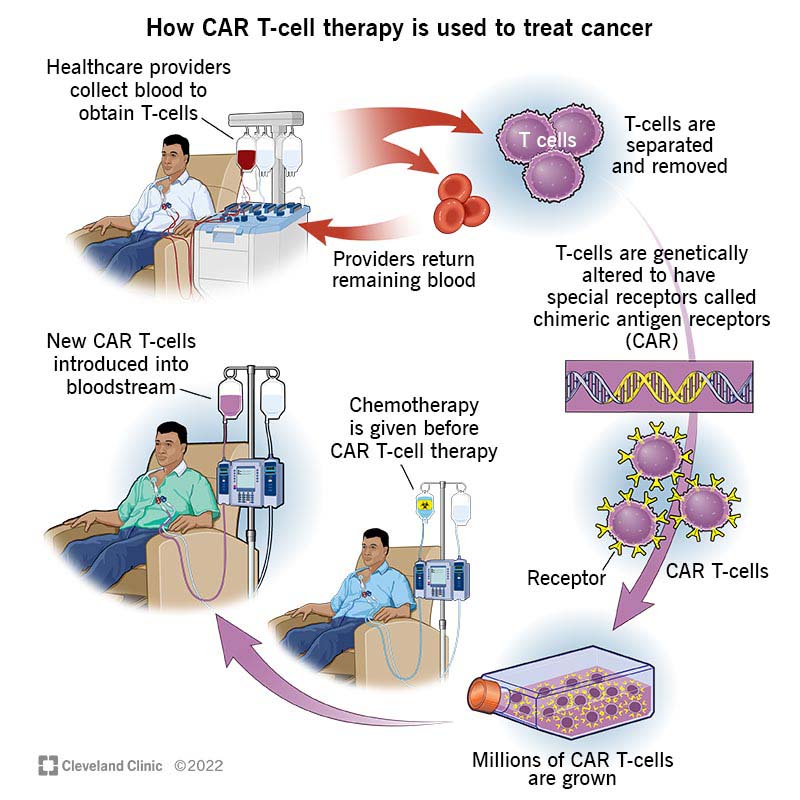
Specialized cell therapies represent a transformative approach in oncology, forging new paths where conventional treatments once reigned. These therapies work by harvesting a patient’s own cells, engineering them to target cancer cells, and reinfusing them into the patient. Though the process involves a number of tricky parts and complicated pieces, it also holds the promise of long-lasting remission or even a cure in certain cancers.
Understanding TIL and TCR-T Cell Therapies
Autologous tumor-infiltrating lymphocyte (TIL) cell therapy and engineered T cell receptor (TCR)-T cell therapies are among the front-runners in the emerging era of cell-based treatments. These approaches harness the body’s immune system in a targeted manner, and early trials have led experts to suggest that these therapies could offer durable, one-time treatments. Despite their promise, there remain several tangled issues concerning how they might be safely and effectively delivered to patients in different healthcare settings.
Lessons from CAR T-Cell Therapy Deployment
One of the more encouraging developments in cellular therapy has been the gradual shift of CAR T-cell treatments from major academic centers into the realm of community clinics. As practitioners glean insights from this experience, it appears that a similar distribution model may well be reachable for TIL and TCR-T therapies. Already, CAR T-cell treatments are known to be “moving more toward community centers,” indicating that the logistical and operational models are becoming accessible even outside high-resource environments. This evolution suggests that, with careful planning, newer cell therapies could follow a similar path, thereby broadening access and potentially improving survival outcomes for many cancer patients.
Challenges and Tricky Parts in Broadening Access
While the future of specialized cell therapies is undoubtedly bright, the journey toward widespread clinical adoption is filled with tricky parts and tangled issues. Key challenges include scaling the sophisticated manufacturing processes, ensuring stringent safety measures outside major research institutions, and training community oncology teams to manage these advanced therapies.
Scaling Up Manufacturing and Quality Control
One of the most complicated pieces of expanding cell therapies is the requirement for specialized manufacturing facilities. Comprehensive cancer centers, such as the renowned Rutgers Cancer Institute, have established Good Manufacturing Practices (GMP) infrastructures that facilitate the rapid translation of scientific findings directly to the patient’s bedside. However, duplicating these resources in a community clinic setting is an intimidating prospect. The current approach requires rigorous quality control and adherence to established protocols to ensure that the cellular products are both effective and safe.
Key Considerations for Manufacturing
- Strict adherence to GMP standards
- Investment in specialized infrastructure
- Training of technical and clinical staff
- Regulatory oversight and ongoing monitoring
These considerations highlight the fine points and hidden complexities involved in moving from a centralized model to a more decentralized, community-based approach.
Managing Safety and Efficacy in Community Settings
The safe administration of cell therapies outside of tertiary centers presents its own set of challenges. Community providers must be equipped not only with the technical know-how to handle these products but also with systems to manage potential side effects and complications. While the tracking of adverse events is already well-established in major centers, replicating such monitoring in less specialized settings might prove nerve-racking at first.
Safety Protocols and Training
| Protocol Area | Key Components |
|---|---|
| Pre-treatment Evaluation | Patient selection, molecular testing, baseline health assessments |
| Manufacturing Oversight | Quality control, sterile processing, regulatory compliance |
| Post-infusion Monitoring | Adverse event tracking, follow-up imaging, lab tests |
| Staff Training | Emergency protocols, infusion techniques, supportive care procedures |
Developing robust safety protocols and investing in comprehensive training programs will be essential to help community physicians make their way through these tricky parts and deliver treatments that are both effective and safe.
Transitioning from Research Centers to Community Clinics
The transition from specialized research centers to broader community adoption is not an overnight endeavor, but the current trends indicate encouraging progress. The model used in CAR T-cell therapy deployment offers a roadmap for how TIL and TCR-T cell therapies could be integrated into routine cancer care.
Building the Necessary Infrastructure
Establishing the infrastructure needed to support specialized cell therapies in community settings is as critical as it is challenging. Typically housed in large cancer centers, the clinical trials and manufacturing processes have been supported by substantial investments in technology and personnel. For a successful move towards community medicine, several fine points must be considered:
- Investment in state-of-the-art laboratory and production facilities
- Partnerships between community clinics and established research institutions
- Adoption of telemedicine and remote monitoring technologies
- Streamlined regulatory processes that maintain safety without being overly burdensome
Each of these elements plays a key role in ensuring that patients can eventually receive complex cell therapies in settings that are closer to home.
The Role of Collaborative Networks
To alleviate the intimidating prospect of independently managing cell therapy programs, community clinics might benefit from forming collaborative networks with comprehensive cancer centers. Such partnerships can help community practices figure a path through the often overwhelming maze of regulatory, scientific, and clinical challenges. By sharing resources, experiences, and training modules, these alliances create a support system that benefits all involved parties.
For example, a network might consist of:
- Research mentoring programs where experienced oncologists help community physicians
- Shared access to manufacturing facilities through satellite laboratories
- Joint clinical trials and data sharing agreements
- Regular teleconferences and workshops on best practices
Such collaborative measures are not just practical—they are essential for the broader adoption of these innovative therapies.
Patient-Centered Perspectives and Community Impact
The potential transition from exclusive academic settings to community clinics is exciting for patients, who often face overwhelming challenges when traveling long distances for specialized care. Integrating advanced cell therapies into local practices has the potential to transform patient experiences by reducing travel burdens and fostering a sense of community-based care.
Improved Access and Patient Convenience
Patients living in remote or underserved areas frequently encounter off-putting barriers when seeking specialized cancer treatments. By making cell therapies available locally, the healthcare system can begin to address these issues effectively. The following bullet list summarizes the key benefits for patients:
- Reduced travel time and expenses
- Easier access to follow-up care and monitoring
- Increased opportunities for quicker treatment initiation
- Improved comfort and familiarity with local healthcare providers
These factors combined can lead to a more patient-friendly experience, potentially contributing to better outcomes and higher overall satisfaction.
Empowering Local Oncologists
Another critical benefit of expanding cell therapy access is the empowerment of community-based oncologists. When local clinicians are granted the tools and training necessary to administer these treatments, they are better positioned to make informed, patient-centered decisions. This empowerment not only enhances the quality of care but also builds trust within the community. Local healthcare providers can:
- Develop tailored treatment plans based on a deep understanding of local patient demographics
- Participate in clinical research initiatives that address region-specific challenges
- Offer a continuum of care that extends from diagnosis to long-term follow-up
Such an environment fosters a culture of continuous improvement and shared expertise, paving the way for more innovative and customized treatment solutions.
Fine-Tuning the Transition: The Role of Policy and Regulation
As promising as the expansion of cell therapies into community settings may be, it is also loaded with policy challenges and regulatory twists and turns. The regulatory framework must evolve to address the subtle details of decentralized manufacturing and clinical administration without compromising patient safety.
Reforming Regulatory Processes
Government agencies and regulatory bodies play a super important role in ensuring the safety and efficacy of cell therapies. Streamlining the approval process, reducing bureaucratic delays, and implementing adaptable guidelines for both academic and community settings are all critical steps. Some proposals include:
- Establishing pilot programs that allow for controlled community-based administration of cell therapies
- Developing risk-based regulatory models that can be calibrated according to the treatment setting
- Introducing incentives and support systems for community clinics to invest in necessary infrastructure
These suggestions offer a glimpse into how thoughtful policy changes could ease the nerve-racking challenges associated with adopting these advanced treatments in a broader landscape.
The Role of Public-Private Partnerships
Public-private partnerships may also serve as a catalyst in overcoming the intimidating regulatory and logistical barriers. By combining the resources of government bodies, academic institutions, and private companies, these ventures can accelerate the process of bringing novel cell therapies to wider audiences. Benefits of such partnerships include:
- Shared expertise in manufacturing and technology
- Collaborative funding for research and infrastructure development
- Streamlined information exchange and knowledge dissemination
These alliances have the potential to reduce overhead costs, minimize the learning curve for community providers, and ultimately translate innovation into improved patient care.
Economic Considerations in Expanding Cell Therapies
Beyond the clinical and regulatory challenges, the economics of expanding cell therapies into community settings are also full of tricky parts. It is critical that all stakeholders—clinicians, administrators, payers, and policymakers—work together to ensure that these treatments are both effective and cost-efficient.
Evaluating Cost-Effectiveness and Reimbursement Models
One of the overwhelming factors when considering the local adoption of advanced therapies is the economic viability of scaling up such treatments. While the initial development and manufacturing costs may be high, strategic investments and innovative reimbursement models could help mitigate these financial burdens. For example:
- Bundled payment models that cover the entire treatment cycle
- Risk-sharing agreements between manufacturers and insurers
- Government grants and subsidies for community clinics investing in new infrastructure
By carefully designing reimbursement strategies that align with patient outcomes and long-term savings, stakeholders can better ensure that the benefits of cell therapies are both clinically and economically sustainable.
Long-Term Economic Benefits for Communities
Expanding access to these therapies might also lead to broader economic benefits for local communities. Improved patient outcomes often correlate with a reduction in long-term healthcare costs. Furthermore, local administration of advanced treatments can keep healthcare spending within the community, thereby stimulating local economies. Some potential economic advantages include:
- Lower travel and logistical costs for patients and families
- Enhanced local employment as clinics expand and new roles are created
- Increased economic activity through improved health outcomes and reduced disability
The economic ripple effects of localized advanced cancer care could prove to be significant, and they underscore the importance of making these treatments more widely accessible.
Looking Ahead: The Future of Cell Therapy in Community Oncology
The vision of taking cutting-edge cell therapies from specialized, central cancer centers to community clinics is both inspiring and challenging. Experts such as Dr. Christian S. Hinrichs, who have dedicated their careers to advancing these treatments, offer a measured optimism that inspires further innovation and collaboration. While predicting the future remains tricky and filled with unpredictable twists and turns, current trends strongly suggest that the path taken by CAR T-cell therapies provides a reliable roadmap for TIL and TCR-T cell therapies.
Steps Toward a Broader Adoption
For these treatments to successfully make the transition into community settings, the medical community must engage in ongoing dialogue, research, and collaborative practice. Key steps moving forward include:
- Continuing multi-center clinical trials to validate safety and efficacy in diverse settings
- Developing educational programs aimed at empowering community oncologists
- Investing in new infrastructure that meets GMP standards while remaining economically viable
- Advocating for policy reforms and streamlined regulatory processes
Each of these steps requires careful attention to the fine points and subtle differences between academic and community practices. Nevertheless, the potential to transform cancer care on a wide scale makes tackling these challenges well worth the effort.
Collaborative Research and Data Sharing
One promising avenue for smoothing the transition is increased collaboration between academic centers and community clinics. This collaboration goes beyond sharing best practices—it involves joint research, data sharing, and open communication. Establishing a central repository for real-world data on cell therapy outcomes could help clinicians from all environments learn from one another, adapting strategies in real time to improve both safety and efficacy.
By pooling their collective experience, community physicians can get around some of the intimidating challenges of incorporating these advanced therapies into everyday practice. This cooperative approach not only enhances patient care but also accelerates innovation across the board.
Patient Stories and Community Testimonials
No conversation about the future of cell therapy would be complete without the real-life accounts of patients and local healthcare providers who have firsthand experience with these treatments. Stories emerging from early clinical trials and community-based initiatives are powerful testimonials to the transformative potential of these therapies.
Overcoming the Nerve-Racking Waiting Game
Patients often describe the period between diagnosis and the start of treatment as an overwhelming and nerve-racking time. The ability to receive state-of-the-art care closer to home can dramatically shorten this waiting period, providing much-needed psychological relief and practical benefits. Local access to specialized therapies allows patients to:
- Receive prompt care with minimal delay
- Maintain closer contact with their support networks
- Avoid the additional stress of long-distance travel
- Experience care from providers who understand local community dynamics
These benefits, though sometimes subtle, contribute to a more positive overall treatment experience and can have a significant impact on long-term outcomes.
Local Oncologists Share Their Expert Insights
Community oncologists who have started adopting cell therapies in their practices emphasize both the promises and the challenges that come with these treatments. They note that while the technical parts of delivering these innovative therapies can be tricky, the rewards in terms of patient survival and quality of life are immense. As one oncologist commented:
“Working with these novel cell therapies is like learning a new language. The early days are full of off-putting technical details and nerve-racking complications, but each success story helps us better figure a path towards more widespread, secure, and effective treatment strategies.”
Such testimonials underscore the critical importance of having on-the-ground expertise and a supportive network that spans from academic research to everyday clinical practice.
Addressing the Nitty-Gritty of Implementation
Implementing such cutting-edge treatments in community settings demands a comprehensive approach that addresses multiple layers of healthcare delivery. Whether it is the education of staff, the adaptation of complex manufacturing processes, or the careful monitoring of patient outcomes, every step involves a series of connected, challenging bits.
Optimizing Clinical Pathways for Better Outcomes
To ensure that patients experience the full benefits of cell therapy, it is essential for healthcare providers to refine clinical pathways and treatment protocols. This involves carefully charting out every twist and turn of the treatment process, from initial screening to long-term follow-up. Key elements include:
- Patient Selection: Identifying the right candidates through personalized diagnostics and molecular testing.
- Treatment Initiation: Setting up quick and efficient pathways to begin cell manufacturing once a patient is approved.
- Post-Treatment Management: Implementing robust monitoring systems to manage potential side effects and complications seamlessly.
- Continuous Feedback: Leveraging patient outcomes and clinical data to refine and optimize protocols over time.
The goal is to create a seamless system where each component talks to the other—ensuring that both the technical and human aspects of care are harmoniously integrated.
Educational Initiatives and Continuous Learning
Given the off-putting learning curves associated with these advanced therapies, continuous education and professional development become super important. Institutions can organize workshops, webinars, and certification programs that help community oncologists dip into the nitty-gritty details of cell therapy. These initiatives may cover:
- Technical training on cell collection, processing, and infusion techniques
- Workshops on managing immune-related adverse events with practical case studies
- Insightful discussions on evolving guidelines and best practices from leading research centers
Such educational programs effectively equip clinicians with the knowledge and confidence to get into these treatments and to provide high-quality, patient-centered care.
Final Thoughts: A Promising Future on the Horizon
The journey to bring advanced cell therapies out of specialized cancer centers and into community clinics is complex, filled with tricky parts and tangled issues. Yet, as we witness the gradual yet steady shift of CAR T-cell treatments into local settings, the future for TIL and TCR-T therapies appears equally promising. With continued collaboration between academic leaders, community clinicians, and policymakers, the dream of offering these life-saving treatments to a broader population is steadily becoming a reality.
While there are still many technical and regulatory hurdles to overcome, the potential benefits—reduced patient travel burdens, local access to advanced care, and ultimately improved clinical outcomes—are too significant to ignore. By working together, the healthcare community can steer through the overwhelming challenges and ensure that the next generation of cancer treatments is not confined to the walls of major research centers, but is instead accessible to every patient in need.
A Roadmap for Future Progress
In summary, practical steps moving forward include:
- Investing in infrastructure upgrades for community clinics to meet GMP standards
- Strengthening public-private partnerships to foster innovation and shared learning
- Implementing robust training programs for local oncologists and support staff
- Reforming regulatory guidelines to better suit decentralized treatment models
- Developing collaborative networks that enable real-time data sharing and peer support
Each of these steps represents a critical piece of the larger puzzle—a puzzle that, once solved, will redefine the landscape of cancer care for generations to come.
Concluding Reflections
The potential to revolutionize cancer treatment through specialized cell therapies is both exciting and within reach. While the path forward is laden with intimidating challenges—from the technical twists and turns in manufacturing to the nerve-racking prospects of decentralized care—the rewards are immeasurable. Patients deserve access to the latest medical advancements without the burden of logistical and geographical barriers, and community clinics are uniquely positioned to make this a reality.
Dr. Hinrichs and many other thought leaders in the field have offered invaluable insights into how this transition might unfold. Although the journey involves navigating a maze of regulatory details, infrastructure demands, and training needs, the commitment of the medical community to finding a path through these puzzling bits is unwavering. With coordinated effort and sustained innovation, the next decade could transform the way we approach cancer care, making advanced therapies a tangible reality for patients everywhere.
As we look ahead, the importance of staying agile and adaptive cannot be overstated. Clinicians, administrators, and policymakers must continue to work together with the shared goal of improving patient outcomes. With each step forward, the vision of a healthcare system where cutting-edge treatments are not always out of reach is coming closer to fruition. Today’s challenges offer a glimpse of tomorrow’s breakthroughs—a hopeful reminder that, even amid complicated pieces and overwhelming obstacles, the promise of life-saving treatments is within our grasp.
In our rapidly evolving landscape, it is crucial to remain committed to innovation, collaboration, and patient-centered care. The future of cell therapy is not just a tale of scientific achievement—it is the story of community resilience, shared expertise, and the relentless pursuit of a better quality of life for all those touched by cancer.
Originally Post From https://www.targetedonc.com/view/dr-hinrichs-on-the-future-of-cell-therapy-access-from-cancer-centers-to-communities
Read more about this topic at
How to democratize cell and gene therapy: A global approach
How to democratize cell and gene therapy: A global …