
Bridging the Gaps in Lung Cancer Care: A Call for Rethinking Post-Immunotherapy Strategies
Over the past decade, immunotherapy has reshaped the treatment landscape for non–small cell lung cancer (NSCLC). Despite these advances, one persistent problem looms large in clinical practice—the lack of effective treatment options once patients progress after immunotherapy. As clinicians continue to confront this challenge, it is clear that the current standard of care—which predominantly relies on docetaxel, alone or in combination with anti-angiogenic drugs—falls short of addressing the multifaceted needs of these patients.
Tackling the Tricky Parts of Post-Immunotherapy Treatment for NSCLC
One of the most pressing issues in lung cancer treatment today is what happens after a patient’s disease progresses beyond immunotherapy-based regimens. Presently, docetaxel remains the backbone of post-immunotherapy treatment, sometimes combined with anti-angiogenic agents, such as VEGFR inhibitors. However, significant efforts aimed at changing this standard have not been successful, leaving clinicians and patients struggling with a treatment that often feels both intimidating and limited.
In clinical practice, the choice between using docetaxel alone or with an anti-angiogenic drug is largely dictated by local reimbursement policies and national treatment standards. This reliance on cost and policy rather than on robust clinical evidence means many patients may not receive the most ideal treatment following progression on immunotherapy.
Exploring the Confusing Bits of Standard Chemotherapy and Its Limitations
For many oncologists, switching to docetaxel after immunotherapy failure is akin to moving from one challenging treatment scenario to another. Despite its long history of use, docetaxel offers only moderate benefits in terms of overall survival and quality of life. The side effects can be overwhelming, and the drug’s efficacy is frequently called into question when compared to the dramatic early successes seen with immunotherapy. This juxtaposition creates a clinical conundrum—how can we effectively treat patients when the only reliable option remains a therapy that has significant limitations?
The limited efficacy of docetaxel and its combinations has spurred the medical community to search for alternative solutions. However, past attempts, notably those involving VEGFR tyrosine kinase inhibitors (TKIs), have consistently failed to demonstrate sufficient superiority. Even the idea of “resensitizing” tumors to immunotherapy by introducing another drug has not yet panned out, leaving a sizeable gap in treatment strategies.
Late Disease Progression: The Nitty-Gritty Details of a Persistent Challenge
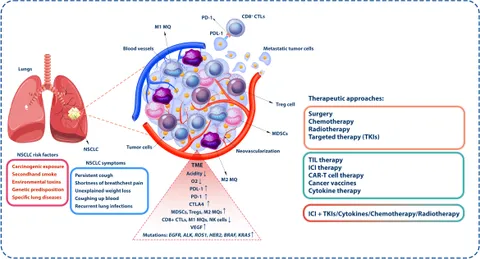
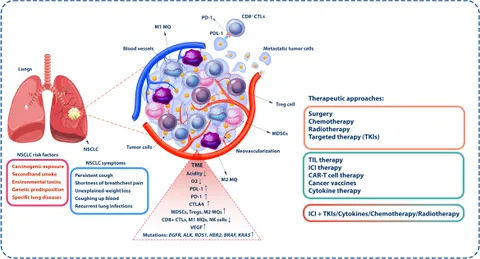
One of the most intricate challenges facing oncologists is the issue of treating patients who experience late disease progression after receiving immunotherapy. The underlying hypothesis is that a tumor’s sensitivity to immunotherapy might be reinstated if a different therapeutic agent is introduced at the right moment. In theory, this approach could reawaken the immune system’s ability to attack cancer cells.
However, the real-life application of this concept is riddled with problems. The complexity of tumor biology means that the interactions between immune cells and cancer cells are influenced by a host of confusing bits—ranging from the microenvironment to genetic mutations. Despite efforts to dig into these subtle parts of tumor behavior, clinical trials involving VEGFR TKIs have not yet succeeded in delivering promising results. The outcome is a widespread sense of frustration among clinicians, who feel that the potential of reintroducing immunotherapy remains unfulfilled.
Lessons from Global Consensus Meetings: A Broad Perspective on the Issue
At the 2025 Global Bridging the Gaps in Lung Cancer consensus meeting, experts from around the world gathered to discuss these pressing issues. One of the key takeaways was the urgent call for new clinical trials that address the unmet needs of post-immunotherapy NSCLC patients. Dr. Maurice Perol from the Léon Bérard Cancer Center in Lyon, France, was among the voices emphasizing the need for innovation. His presentation highlighted that while current therapies are a stopgap, there is a pressing need to develop novel strategies that go beyond traditional chemotherapy.
Consensus from the meeting underscored that the current treatment paradigm is not only off-putting due to its limited effectiveness but also does not fully address the complexities encountered in everyday clinical practice. The conversation stressed that to overcome these tangled issues, research must pivot from incremental changes to bold, innovative approaches that target the disease at its core.
Understanding the Key Factors Behind Treatment Failure
The failure of past efforts, particularly those using VEGFR TKIs, points to several underlying issues. First, the biology of NSCLC is full of twists and turns that make it difficult for a single treatment modality to be universally effective. Cancer cells are notorious for their ability to adapt, often employing alternative pathways to survive even when one treatment avenue is blocked.
Moreover, the fine details of how immunotherapy interacts with the tumor microenvironment add an extra layer of complexity. There are subtle parts of tumor biology that remain poorly understood, making it challenging to evaluate why certain tumors may or may not be resensitized by subsequent treatments. As researchers continue to poke around these issues, the need for deeper molecular insights becomes all the more critical.
- Resistance Mechanisms: Cancer cells can activate alternative signaling pathways that bypass the effects of docetaxel and other cytotoxic therapies.
- Tumor Heterogeneity: Variations within tumor cells limit the overall effectiveness of uniform treatment strategies.
- Microenvironment Factors: The tumor’s surrounding environment may inhibit the immune system’s ability to mount a successful attack once immunotherapy resistance develops.
Understanding these fine points is essential for developing smarter, more personalized treatment approaches that can offer relief to patients who currently have limited options.
Examining the Role of Clinical Trials in Paving New Treatment Paths
The lessons learned from various failed attempts highlight the need for robust clinical trials to assess new combinations and sequences of treatment. One promising initiative is the phase 3 LATIFY study, designed to compare ceralasertib plus durvalumab (Imfinzi) with docetaxel in patients who have progressed after anti–PD-(L)1 therapies and platinum-based chemotherapy.
Clinical trials like LATIFY are key to finding new pathways in a landscape that is typically full of problems. They offer hope that a combination of novel agents can overcome the limitations of traditional treatments and ultimately pave the way for improved patient outcomes. Importantly, these trials also serve as a platform for understanding the confusing bits of tumor response and the fine details that determine treatment success or failure.
Why the Current Standard Falls Short: A Closer Look at Docetaxel
There is no doubt that docetaxel has been a workhorse in lung cancer treatment for years. Its predictable mechanism of action offers some stability in a field characterized by rapid changes. However, its benefits are modest at best. Given that many patients viewed immunotherapy as a turning point in their treatment journey, the subsequent reversion to a less effective chemotherapy regimen can seem like a regressive step.
Beyond its modest efficacy, the side effects associated with docetaxel can be overwhelming. Patients often experience an array of toxicities that compromise their quality of life. The cycle of initial hope with immunotherapy followed by the disappointment of limited options after progression creates both a psychological and physical burden on patients, making the search for better treatments all the more critical.
Exploring Alternative Therapies: The Promise and Pitfalls
In the quest for alternatives, several approaches have been explored. One line of investigation has involved the use of antibody-drug conjugates (ADCs). Initially developed for the post-chemoimmunotherapy setting, ADCs were once seen as a potential game changer. However, their development is increasingly focused on front-line applications, leaving little room to address the unmet need in the post-immunotherapy setting.
Nonetheless, ADCs have not been entirely discounted. Their ability to deliver targeted therapy directly to cancer cells is an essential innovation that holds promise for the future of NSCLC treatment. For now, though, the main challenge remains: finding treatments that can be effectively implemented after progression on immunotherapy. The possibility of tumor resensitization still holds theoretical promise, but translating that into clinical practice will require overcoming several intertwined challenges.
Learning from Past Failures: Anti-Angiogenic Therapies and VEGFR TKIs
Efforts to incorporate VEGFR TKIs into the treatment regimen have been met with mixed results. Despite the rationale behind attacking the tumor vasculature, the results have been underwhelming. The disappointing results highlight the tricky parts of cancer treatment—the hope that targeting one pathway could be sufficient is often dashed by the tumor’s utilization of multiple survival mechanisms.
When treatments fail, it is often not due to a single factor but rather to a combination of tangled issues. These include intrinsic tumor resistance, patient-related factors, and even the timing of drug exposure relative to prior therapies. By studying these failures, clinicians can begin to piece together why certain approaches do not achieve the desired effect, ultimately guiding future research toward more promising strategies.
- Complexity of Angiogenesis: The process by which tumors create new blood vessels is full of small distinctions that influence the overall success of anti-angiogenic drugs.
- Interplay with Immunity: Interrupting the tumor’s blood supply may not always enhance the immune response, creating an unpredictable therapeutic outcome.
- Optimization Challenges: Proper dosing and scheduling are key factors that have yet to be fully refined in clinical settings for these agents.
Patient-Centered Care: Balancing Efficacy with Quality of Life
While the primary focus is often on extending survival, it is equally important to consider the quality of life for patients undergoing treatment. Many patients may view the transition from immunotherapy—which is associated with prolonged periods of disease control and tolerable side effects—to aggressive chemotherapy regimens as a major setback.
The key to improving patient outcomes might lie in developing treatment strategies that not only extend life but also preserve its quality. This necessitates researching novel combinations that minimize toxicity while maximizing efficacy. Engaging patients in discussions about their treatment preferences and realistic outcomes is essential for building a therapeutic relationship grounded in mutual trust and understanding.
Future treatment paradigms should incorporate patient-reported outcomes and quality-of-life measures, ensuring that any new therapy introduced into the post-immunotherapy space is both effective and manageable for everyday living.
Integrating Novel Drug Combinations: Looking Beyond Monotherapy
The failure of monotherapy strategies underscores the importance of exploring drug combinations that might yield a synergistic effect. Combining drugs that work through different mechanisms could address various facets of tumor survival, potentially overcoming the resistance seen with single-agent therapies.
For example, emerging studies are evaluating the combination of DNA damage response inhibitors, like ceralasertib, with immune checkpoint blockers such as durvalumab. This approach is designed to tackle both the genetic underpinnings of tumor growth and the immune system’s ability to recognize and attack cancer cells. While the science behind these mechanisms is loaded with issues, the hope is that a multi-pronged approach may provide a significant breakthrough in treating NSCLC post-immunotherapy progression.
Key considerations when designing these combination studies include:
- The timing of administration to maximize the potential for tumor resensitization.
- The dosing regimen to ensure that toxicities are minimized while therapeutic benefits are maximized.
- Patient selection criteria, incorporating molecular and genetic profiling to tailor treatments to those most likely to benefit.
Expanding Our Understanding: The Need for Biomarker-Driven Approaches
One significant barrier in developing effective post-immunotherapy strategies is the lack of predictive biomarkers that can accurately guide treatment decisions. Biomarkers have the potential to unlock insights into tumor heterogeneity and patient-specific factors, helping clinicians figure a path through complex treatment landscapes.
By isolating the subtle details that predict response or resistance, researchers can better tailor therapies to the individual. Biomarker-driven approaches could identify which patients might respond to a reintroduction of immunotherapy and who might benefit more from a novel drug combination. This personalized approach to medicine is not without its challenges, as it requires rigorous validation and integration of complex datasets from both clinical and laboratory studies.
Incorporating these biomarker strategies into clinical practice may help steer through the current limitations of standard therapies and open up new avenues for patient care. The journey toward precision medicine in lung cancer treatment is both exciting and riddled with challenges, but it is undoubtedly a critical step forward.
Looking Ahead: The Future of Post-Immunotherapy NSCLC Treatment
Despite the obstacles and the history of setbacks, there is a palpable sense of optimism in the oncology community regarding the future of NSCLC treatment. Efforts to integrate novel agents and to design smarter clinical trials are in full swing. The coming years may well usher in a new era of treatment paradigms that move beyond the limited scope of docetaxel-based regimens.
Future research will likely focus on multi-agent approaches that combine the strengths of various therapeutic classes. In particular, the integration of checkpoint inhibitors with targeted therapies and even ADCs represents a promising direction. Early-phase studies are beginning to reveal promising signals, though it is clear that much work remains to be done before these approaches can be widely adopted in clinical practice.
To accelerate progress, it is essential that funding bodies, regulatory agencies, and clinical researchers collaborate closely. Streamlined trial designs, increased patient enrollment, and international cooperation can all contribute to a more rapid evolution of treatment strategies. This collaborative model of research is especially critical in an era where lung cancer remains one of the deadliest cancers worldwide.
Real-World Implications: The Impact on Patients and Healthcare Providers
For patients with advanced NSCLC, the period following progression on immunotherapy is often a time of uncertainty and anxiety. The shift back to an older, less effective standard of care can feel like losing hard-won ground. Oncologists, too, face the daunting task of managing a treatment landscape that is not only limited but also complicated by the patient’s prior exposure to cutting-edge therapies.
The real-world implications of these treatment gaps extend beyond clinical outcomes. Patients grapple with physical side effects, emotional distress, and a disrupted quality of life. Healthcare providers, meanwhile, must continually adapt to emerging evidence and evolving treatment protocols while ensuring that patient care remains compassionate and comprehensive.
In this context, the push for better treatment strategies is not just a scientific or clinical imperative—it is a moral one. Improving post-immunotherapy outcomes is critical not only for extending life but also for enhancing its quality. As the field advances, incorporating patient voices and experiences into treatment decisions will be key to building a more empathetic and effective model of care.
Policy and Reimbursement: Overcoming Systemic Barriers to Innovation
Another layer of complexity in this issue is the role of healthcare policy and reimbursement strategies. In many cases, the choice of treatment is influenced more by what is reimbursed than by what is clinically ideal. This disconnect between policy and practice has significant implications for patient care.
For innovative treatments to reach clinical reality, there must be an integrated approach that aligns regulatory approvals and reimbursement policies with the advancing science. This includes updating guidelines to reflect new evidence, streamlining the approval process for promising novel combinations, and ensuring that financial constraints do not limit patient access to state-of-the-art care.
Policymakers and healthcare administrators need to consider the long-term benefits of investing in research and development for alternative post-immunotherapy strategies. By prioritizing innovative approaches, health systems can reduce the reliance on traditional chemotherapy agents like docetaxel, thereby potentially improving patient outcomes and reducing overall healthcare burdens.
International Collaboration: Sharing Data and Best Practices
The global nature of medical research presents both a challenge and an opportunity. Data gathered from diverse populations across various countries can provide invaluable insights into the effectiveness of different treatment regimens. International collaborations, such as consensus meetings on lung cancer, help bring together the collective wisdom of experts from around the world.
Such collaborations allow for the pooling of resources, sharing of real-world experiences, and the development of standardized protocols that can be tested across a broad spectrum of patients. The coming years will likely see an increase in multinational clinical trials that aim to evaluate novel post-immunotherapy strategies. This concerted effort will be essential if the oncology community is to overcome the current gaps in treatment.
- Data Sharing: International registries and collaborative studies can accelerate the validation of new biomarkers and treatment combinations.
- Harmonization of Treatment Standards: Aligning treatment protocols across different healthcare systems will help ensure that all patients have access to the most effective therapies.
- Cross-Border Clinical Trials: Multinational trials can provide robust evidence that supports the adoption of new treatment standards globally.
Innovative Approaches for a Changing Landscape
Looking forward, several innovative directions stand out as particularly promising. One area of focus is the potential use of next-generation immunotherapies that build on the success of current checkpoint inhibitors while addressing their limitations.
Another exciting avenue is the development of personalized vaccines and adoptive cell therapies, which are designed to harness and boost the patient’s own immune response more effectively. Though these strategies are still in the experimental phase, they represent a shift toward a more individualized approach to cancer care that could be transformative for patients with advanced NSCLC.
Moreover, advances in genomics and proteomics continue to reveal the hidden complexities of tumor biology, offering new targets for therapy. These discoveries underscore the importance of integrating precision medicine into lung cancer treatment, where therapies are tailored to the unique genetic profile of each patient’s tumor.
Ultimately, the path forward will require a blend of new drug combinations, improved diagnostic tools, and a greater emphasis on the patient’s overall well-being. To truly bridge the gaps in lung cancer care post-immunotherapy, we must embrace these innovative approaches while simultaneously refining our understanding of the disease’s tricky parts and subtle details.
Conclusion: A Roadmap to a More Promising Future
The journey from the current state of post-immunotherapy treatment in NSCLC to a future where patients enjoy more effective, personalized, and compassionate care is undoubtedly challenging. However, the need for progress is undeniable. Clinicians, researchers, policymakers, and patients must work together to address the overwhelming gaps that exist in current treatment paradigms.
While docetaxel remains a fallback option today, tomorrow’s landscape could be dramatically different. By fostering international collaboration, embracing biomarker-driven research, and supporting innovative clinical trials, the oncology community can begin to figure a path forward through the tangled issues of current treatment strategies.
This transformation will not occur overnight. It will require sustained commitment, creative thinking, and a willingness to experiment with new approaches—even when the early results may be nerve-racking or the outcomes uncertain. The ultimate goal is to provide NSCLC patients with treatment strategies that not only control the disease more effectively but also improve their quality of life.
As we take a closer look at the evolving field of cancer care, it is clear that the post-immunotherapy phase represents both a significant challenge and a fertile ground for innovation. The lessons learned thus far must be used to guide the design of future therapies. With careful research, patient-centered focus, and international cooperation, we can hope to see a day when the gaps in lung cancer care are not so wide, and where every patient has access to treatments that are both effective and humane.
In closing, the current limitations in post-immunotherapy NSCLC treatment are a powerful reminder of the need to continually adapt and improve our approaches to cancer care. The drive to find solutions to these overwhelming problems must remain unyielding. It is a call to action for all stakeholders in the healthcare community—a call to push forward despite the tangled issues and confusing bits, ever committed to the well-being of those affected by this challenging disease.
Originally Post From https://www.onclive.com/view/dr-perol-on-unmet-need-for-post-immunotherapy-strategies-in-lung-cancer
Read more about this topic at
Addressing the treatment gap: A key challenge for …
Understanding and Addressing the Treatment Gap in …