Assessing the Updated EULAR Guidelines on Antirheumatic Drugs in Reproductive Health
The recent update from the European Alliance of Associations for Rheumatology (EULAR) on antirheumatic drug use in reproductive settings has reignited debates among healthcare professionals. With a focus on managing rheumatic and musculoskeletal diseases (RMDs) during family planning, pregnancy, and lactation, the guidelines aim to help providers better figure a path through the tricky parts of patient management. In this opinion piece, we take a closer look at the revised recommendations, discuss their practical implications, and offer insights into managing both maternal health and fetal safety in everyday practice.
Balancing Disease Management and Reproductive Safety
The updated guidelines stress the importance of balancing treatment efficacy with potential risks to conception and fetal development. One of the key takeaways is that antirheumatic drugs must be carefully chosen based on their safety profile in pregnant and breastfeeding patients. While conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) like hydroxychloroquine, sulfasalazine, azathioprine, and colchicine remain part of the safe arsenal, the recommendations advise caution with drugs known to be harmful, such as cyclophosphamide, methotrexate, and mycophenolate.
The recommendations are clear that treatment should not be an off-putting barrier to family planning. Instead, they stress that timely and consistent reproductive counselling can help patients manage the confusing bits of their treatment plan while minimizing risks associated with untreated maternal disease. The idea is to empower patients to take an active role in discussing treatment strategies with their healthcare providers—a move that is key in today’s evolving clinical landscape.
Enhanced Emphasis on Breastfeeding and Shared Decision-Making
One of the most notable changes in the 2024 EULAR update is the reinforced support for breastfeeding while on compatible antirheumatic medications. In previous versions, there was a tendency to steer clear of breastfeeding until treatment ceases or is switched; however, the new guidance highlights the many benefits of breastfeeding both for the mother and the baby. The updated principle suggests that women should not be discouraged from breastfeeding when they are on medications that are documented as safe. The positive emphasis on shared decision-making means that treatment choices are made together by the patient and the provider, ensuring that the recommendations suit the patient’s individual circumstances.
This shift towards encouraging breastfeeding reflects a broader move in modern medicine, where the controversial twists and turns of treatment decisions are addressed through collaborative planning. Increased dialogue can help manage your way through the small distinctions and hidden complexities of antirheumatic drug therapy during lactation.
Drug Safety During Pregnancy: A Closer Look at csDMARDs and bDMARDs
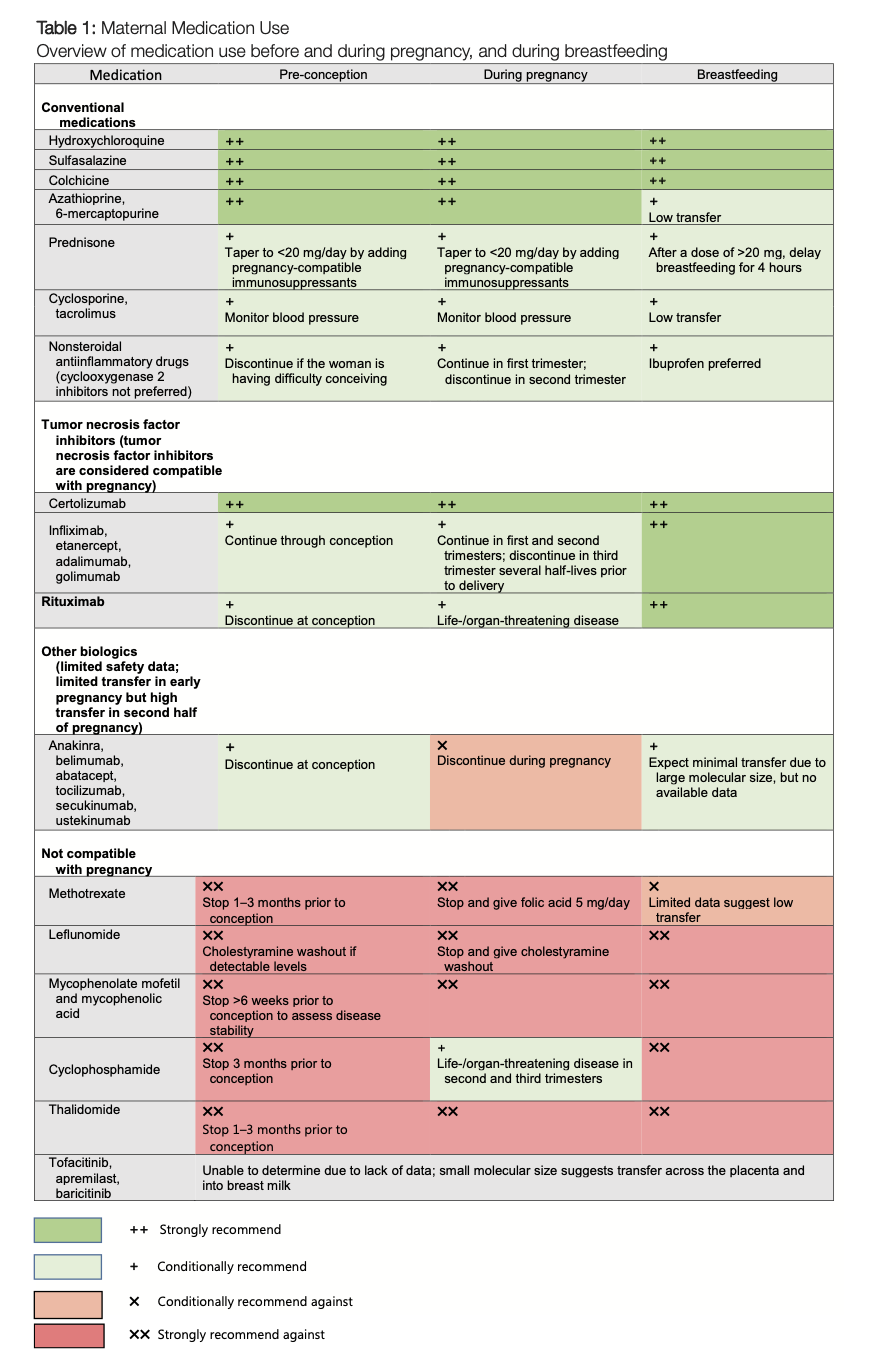
The updated guidance divides drugs into categories based on their safety profiles during the preconception phase, pregnancy, and postpartum periods. For conventional synthetic DMARDs, several key points are made:
- Compatible medications: Hydroxychloroquine, sulfasalazine, azathioprine, and colchicine are recommended as safe for women who are planning pregnancy or are already pregnant. These drugs have not shown any increase in adverse fetal outcomes.
- Contraindicated medications: Cyclophosphamide, methotrexate, and mycophenolate remain off-limits during pregnancy due to their known teratogenic effects. The guidelines outline specific timelines for discontinuing these drugs before conception.
The updated recommendations also take on the tricky parts of using nonsteroidal anti-inflammatory drugs (NSAIDs) and glucocorticoids. NSAIDs are advised for intermittent use and should be discontinued after 28 weeks of gestation. Meanwhile, low-dose prednisone or prednisolone is considered acceptable, but providers are encouraged to taper the dose to 5 mg per day whenever possible.
Biologic DMARDs (bDMARDs) have undergone a significant review. Their use is now considered acceptable during all trimesters of pregnancy, particularly the tumor necrosis factor (TNF) inhibitors. Other non-TNF agents, such as rituximab, abatacept, and tocilizumab, may be utilized if their benefits outweigh the placental transfer risks. However, the guidelines advise caution with newer agents like anifrolumab, mepolizumab, and risankizumab, suggesting that these should only be considered when no safer alternatives exist.
Comparative Overview: Safe and Unsafe Medications During Pregnancy
To clearly sort out the safe options from the intimidating list of contraindicated medications, the chart below summarizes the key points:
| Medication Category | Safe during Pregnancy | Contraindicated during Pregnancy |
|---|---|---|
| csDMARDs | Hydroxychloroquine, Sulfasalazine, Azathioprine, Colchicine | Cyclophosphamide, Methotrexate, Mycophenolate |
| NSAIDs & Glucocorticoids | Intermittent NSAIDs (stop after 28 weeks), Low dose Prednisone/Prednisolone | High dose and continuous NSAIDs use post 28 weeks; high-dose glucocorticoids |
| Biologic DMARDs | TNF inhibitors, Suitable non-TNF agents (Rituximab, Abatacept, Tocilizumab) | Newer agents (Anifrolumab, Mepolizumab, Risankizumab when alternatives exist) |
This table helps providers get around the tangled issues of drug categorization, enabling a clearer pathway for treatment planning during the delicate periods of reproduction.
Individualized Treatment: Weighing the Risks and Benefits
One of the aspects that make clinical decisions feel overwhelming is assessing the twists and turns of treatment risks versus the dangers posed by untreated disease. The updated guidelines insist on a personalized approach that recognizes subtle differences among patients. This means factoring in the severity of the maternal disease, the specific type of antirheumatic medication, and the expected placental transfer rates of these medications.
A nuanced approach involves evaluating:
- The extent of disease activity
- Previous treatment responses
- Individual reproductive goals
- The latest safety evidence on drug use during pregnancy
This tailored strategy can be a great asset when trying to sort out the little details and fine shades of decision-making in reproductive health management.
Breastfeeding Under Antirheumatic Treatment: New Insights
Historically, the compatibility of antirheumatic medications with breastfeeding has been a nerve-racking topic. The updated guidance brings a breath of fresh air by expanding the list of drugs considered safe during lactation. csDMARDs like azathioprine, sulfasalazine, and colchicine, along with anti-inflammatory medications such as celecoxib and ibuprofen, continue to be endorsed. More notably, all TNF inhibitors and most non-TNF bDMARDs are now recognized as compatible with breastfeeding. This change is grounded in emerging evidence that highlights minimal transfer of these drugs into breast milk and low oral bioavailability in infants.
For patients facing the delicate issues surrounding lactation therapy, this update offers reassurance. It suggests that with proper monitoring and dose adjustments, new mothers can pursue an effective treatment plan without compromising their ability to breastfeed. This approach reflects a broader trend toward supporting maternal–child bonds while ensuring that disease management remains super important.
Addressing Medication Impact on Male Fertility
A significant addition to the updated guidelines is a dedicated section that focuses on men who are planning conception. In the past, much of the conversation centered on women’s reproductive health, and the effects of antirheumatic drugs on male fertility were less clear. Now, the recommendations underscore that most csDMARDs and bDMARDs appear safe for men, with the exception of sulfasalazine, which may temporarily affect sperm quality. Additionally, cyclophosphamide is highlighted as a major concern due to its potential to cause irreversible infertility.
For men undergoing treatment, it becomes essential to discuss fertility preservation measures before starting drugs known for their adverse effects on reproductive health. Providers are advised to encourage patients to switch to better-studied alternatives when family planning is on the horizon, thus helping them figure a path through these challenging bits of treatment decisions.
Timing and Dosage Adjustments: The Preconception Puzzle
One of the more nerve-racking aspects for clinicians is determining the right time to taper or switch medication regimens before pregnancy. The updated EULAR recommendations pay close attention to the timing of drug discontinuation, particularly relating to teratogenic medications like methotrexate and cyclophosphamide. Providers are encouraged to discuss a clear timeline with patients who are planning to conceive. For example:
- Discontinuing methotrexate several weeks before conception
- Considering alternative therapies that maintain disease control without teratogenic risks
- Monitoring disease activity closely during the transition period
By laying out clear preconception guidelines, the EULAR task force seeks to demystify the complicated pieces involved in making these crucial adjustments. This proactive approach promotes early and regular reproductive counselling, a practice that can ultimately ease the nerve-racking process of planning for pregnancy while managing RMDs.
Managing Infant Vaccinations After In Utero Exposure
The updated recommendations also include detailed guidance on child vaccinations in cases where mothers have been exposed to biologic agents during pregnancy. The consensus is that infants exposed to bDMARDs in utero can receive nonlive vaccines according to standard immunization schedules. However, particular caution is advised with live-attenuated vaccines, such as Bacillus Calmette-Guerin (BCG) and rotavirus. Depending on the timing of exposure and the specific agent used, these vaccines might need to be delayed for up to six months.
This facet of the guidelines emerges from concerns about the subtle differences in an infant’s immune response after prenatal exposure to immunomodulatory drugs. By clarifying vaccination timelines, the recommendations help figure a path through the intricate balance of protecting a child’s developing immune system while not compromising the immunization schedule.
Practical Implications for Clinicians in Everyday Practice
For clinicians working with patients who have rheumatic conditions, these updated guidelines are more than a set of recommendations—they are a roadmap to better patient outcomes. The guidelines urge providers to:
- Encourage early reproductive counselling: Initiate discussions about family planning as soon as possible and integrate these conversations into regular care routines.
- Promote shared decision-making: Work closely with patients to understand their priorities and preferences, thereby easing the tangled issues associated with treatment decisions.
- Tailor treatment plans: Apply individualized strategies that consider both disease control and reproductive safety. Adjust dosages and timing based on the patient’s current health, treatment response, and family planning goals.
- Monitor closely: Keep track of maternal disease activity and fetal health through regular check-ups and updated diagnostic tools.
- Educate about vaccination: Inform parents about any necessary delays in live-attenuated vaccines, ensuring they understand the rationale behind these guidelines.
The overarching message is that managing rheumatic disease during key reproductive stages should not be intimidating or prohibitive. Instead, it should be viewed as a collaborative and personalized process—an opportunity to find your way through the challenging bits and ensure both maternal and fetal well-being.
Weighing the Benefits: A Closer Look at Shared Decision-Making
One of the most commendable aspects of the updated guidelines is the emphasis on shared decision-making. This concept is not just about clinicians assigning strict dosages; it is about engaging in a dialogue that covers the full spectrum of treatment options, potential risks, and expected benefits. In today’s increasingly patient-centered healthcare environment, providers are expected to:
- Explain the fine points of various antirheumatic drugs in simple language.
- Discuss the individual risks related to drug exposure during pregnancy and breastfeeding.
- Co-create a treatment plan that reflects the patient’s unique situation and reproductive plans.
In practice, this means that rather than simply following protocol, physicians take time to get into the fine details with their patients. The approach helps demystify the complicated pieces of treatment decisions and reinforces a trusting relationship, which is especially key when dealing with sensitive issues like reproductive health.
Digging Into the Evidence: How Safety Data Influences Guidelines
It is important to note that the updated recommendations are built on a robust foundation of systematic literature reviews and consensus among international experts. A multidisciplinary task force, comprising 27 experts from 13 countries, meticulously reviewed safety data for antirheumatic drugs in various reproductive contexts. Their method involved assigning a level of evidence and grading the recommendations using the Oxford Centre for Evidence-Based Medicine system.
This structured approach not only adds credibility to the guidelines but also offers reassurance to clinicians. The recommendations are meant to help providers sort out the subtle parts of treatment choices, backed by evidence rather than anecdote. By relying on this data, physicians can make informed decisions and confidently steer through the tricky bits of potential risks and benefits in patient care.
The Role of Patient Education in Implementing New Guidelines
One ongoing challenge is ensuring that patients fully understand the rationale behind these recommendations. Often, the rules governing medication use during pregnancy and lactation can feel overwhelming and even intimidating. Patient education plays a super important role here. By translating these guidelines into simpler language, healthcare professionals can empower patients to join the decision-making process.
Helpful educational strategies include:
- Creating detailed pamphlets that explain which drugs are safe and which are not.
- Holding informational sessions or webinars about managing rheumatic diseases during family planning.
- Using visual aids like flowcharts to illustrate when to taper medications or delay vaccinations.
When patients are informed, they are better positioned to ask questions and take part in creating a treatment plan that minimizes risk while efficiently managing their condition. This educational approach softens the intimidating edges of the recommendations and ensures that both patient and provider are aligned in their goals.
Challenges and Opportunities: The Future of Antirheumatic Drug Use in Reproductive Health
Despite the clarity brought by the updated guidelines, several challenges remain. One of the most tangled issues is the limited safety data available for newer agents, especially for male patients. When data is scarce, clinicians must often work with a degree of uncertainty, which can be nerve-racking. Nonetheless, this opens up opportunities for further research and collaboration among international experts, paving the way for even more refined guidelines in future revisions.
Opportunities for progress include:
- Expanded research: More studies are needed to evaluate the safety of newer biologic agents and small-molecule drugs in both male and female patients during preconception and pregnancy.
- Increased international collaboration: Collaborative research initiatives can help pool data from different populations and provide a more comprehensive safety profile.
- Patient registries: Establishing registries for patients on antirheumatic drugs can help track outcomes and refine treatment protocols over time.
These opportunities point to a future where clinicians may find even greater clarity in the fine details of medication management, easing the confusing bits of decision-making and ultimately leading to better outcomes for both mothers and their children.
Concluding Thoughts: A Collaborative Future in Reproductive Health
The updated EULAR recommendations represent a significant step forward in managing antirheumatic drug use during the sensitive periods of conception, pregnancy, and lactation. By urging early reproductive counselling, promoting shared decision-making, and providing individualized, evidence-based guidelines, these updates help both clinicians and patients manage their way through the small distinctions and tangled issues of treatment planning.
While challenges remain—especially around newer agents and limited male fertility data—the guidelines emphasize a balanced, patient-centered approach that places equal importance on disease control and reproductive safety. In an era where the twists and turns of medical decision-making are increasingly complex, these recommendations provide a clear, evidence-based roadmap for navigating the nerve-racking parts of antirheumatic treatment in reproductive health.
In summary, the updated guidance from EULAR serves as a reminder that effective disease management requires not only scientific rigor but also empathy and open communication between patient and provider. The future of antirheumatic therapy in reproductive health lies in embracing collaborative, individualized care—one that ultimately enhances outcomes and supports the overall well-being of families facing the challenges and rewards of modern medicine.
As we continue to get into the nitty-gritty of these updated recommendations, it is our hope that healthcare professionals worldwide will find these guidelines a useful tool. By working together, we can steer through the intimidating aspects of treatment planning and ensure that every patient receives the most appropriate, safe, and effective care possible.
The changes offered by the 2024 EULAR update are not just academic; they have practical, real-world implications that will impact countless families. By making the somewhat off-putting task of managing complex disease treatment during reproduction a shared journey, we are better equipped to overcome the subtle parts and hidden complexities of antirheumatic therapy in an ever-evolving medical landscape.
Ultimately, these guidelines reaffirm a commitment to patient-centered care—a promise that health professionals will always prioritize the delicate balance between maternal well-being and fetal safety. As this conversation continues to evolve, the hope is that future research, enhanced patient education, and global cooperation will further solidify a treatment framework that truly meets the diverse needs of patients managing rheumatic conditions during their reproductive years.
It is our responsibility as clinicians, researchers, and advocates to use these guidelines as a platform for ongoing improvement in the field of reproductive health. By adopting an approach that is both flexible and evidence-based, we can confidently figure a path through the challenging parts and support patients in one of the most important chapters of their lives—the decision to start or expand a family.
Originally Post From https://www.rheumatologyadvisor.com/features/2024-eular-update-antirheumatic-drugs-in-reproductive-health/
Read more about this topic at
Reproductive Health in Rheumatic Diseases Guideline
EULAR recommendations for use of antirheumatic drugs in …