Rituximab: A Promising New Chapter in Autoimmune Hemolytic Anemia Treatment
In recent years, the medical community has seen a growing interest in the role of rituximab as an innovative solution for autoimmune hemolytic anemia (AIHA), a challenging blood disorder characterized by the body’s misguided attack on its own red blood cells. While traditional treatments such as corticosteroids and immunosuppressants have served as the mainstay for management, many patients continue to face tricky parts and tangled issues like relapses and treatment-related complications. As an editor with deep insights into modern and alternative medicine, I will take a closer look at the emerging evidence that supports rituximab, demonstrating how this therapy stands out as a game-changer for AIHA. This opinion editorial is designed to help readers understand the innovative approach behind rituximab and assess its potential to improve long-term outcomes.
Understanding Autoimmune Hemolytic Anemia in Today’s Medical Landscape
Autoimmune hemolytic anemia is a rare condition with an incidence rate estimated at between 1 and 3 cases per 100,000 people each year. The disorder arises due to the body producing autoantibodies that mistakenly destroy red blood cells. This self-targeting reaction often leads to symptoms that are as confusing as they are concerning, ranging from fatigue and pallor to more severe manifestations like jaundice and splenomegaly. The complexity of AIHA lies not only in its presentation but also in its treatment, as many conventional therapies are laden with issues that may result in relapse or may even become overwhelming for patients over time.
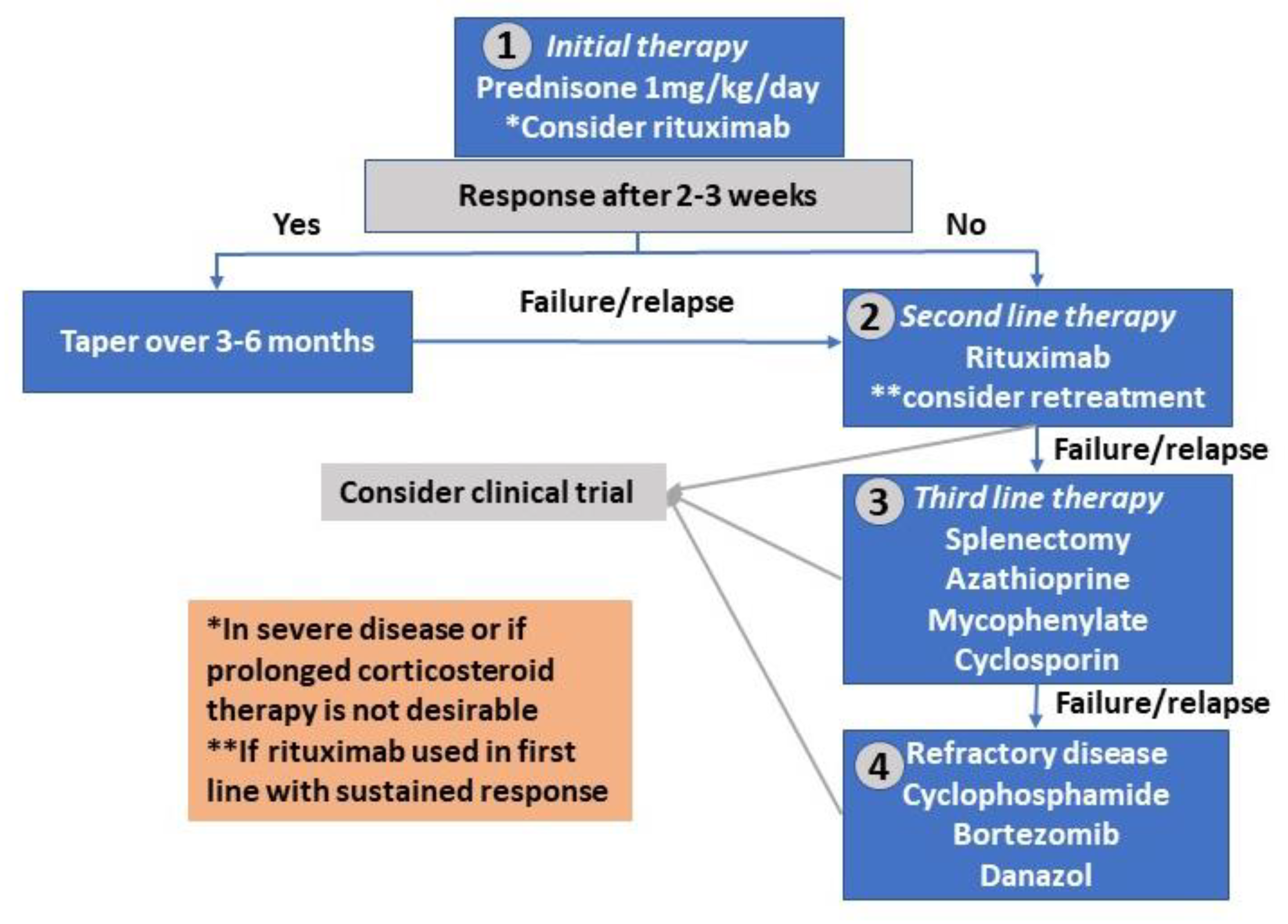
Rituximab, a monoclonal antibody that targets CD20-positive B cells, has emerged as a noteworthy alternative for patients whose conditions have not responded well to corticosteroids. By selectively eliminating B cells, rituximab interrupts the chain reaction of autoimmune destruction, thereby offering hope for sustained remissions—a crucial goal for clinicians who need to make their way through the maze of treatment options for AIHA.
A Closer Look at the Evidence Supporting Rituximab Treatment
A growing body of research has statistically supported the use of rituximab in AIHA, primarily through systematic reviews and meta-analyses. One such meta-analysis incorporated data from 10 high-quality studies to evaluate the long-term efficacy and safety of rituximab compared with conventional therapies. The gathered evidence consistently showed that rituximab improved remission rates, lowered relapse frequencies, and even reduced the number of plasma exchange sessions required to attain remission—a key indicator of clinical benefit.
In many studies, rituximab was administered using one of two dosing protocols: either 375 mg/m² weekly for four consecutive weeks or as two separate doses of 1000 mg. Both schemes have shown promising results in terms of replicating higher ADAMTS13 activity—a critical measure in some autoimmune conditions—and contributing to better patient outcomes by lessening reliance on additional invasive procedures.
Key Findings from Meta-Analyses and Clinical Trials
The meta-analysis outcomes point to a statistically significant benefit of rituximab over traditional treatments:
- Enhanced Remission Rates: Patients receiving rituximab experienced a 2.45-fold higher chance of sustained remission compared to those under conventional treatment protocols.
- Reduced Relapse Incidence: Subgroup analyses revealed that rituximab, especially when used as a second-line treatment after steroid failure, results in fewer relapses—an outcome that provides critical comfort to patients facing confusing treatment decisions.
- Decreased Need for Plasma Exchange: With lower numbers of required plasma exchange sessions, rituximab shows that it can ease some of the burdens of traditional therapies, which are often laden with nerve-racking procedures.
- Stable Safety Profile: Although a small percentage of patients experienced infusion-related reactions (such as chills and fever), these were usually well-managed by premedication strategies. Moreover, severe adverse events, including significant infections, were rare, underscoring the overall benefit-risk balance of this treatment.
These bullet points not only serve to organize the complex details of rituximab’s benefits but also demonstrate how research findings translate into everyday clinical decisions. Tables summarizing patient demographics, dosing regimens, and clinical outcomes further underscore the multidisciplinary effort to optimize therapy in AIHA.
Clinical Implications: What Does Rituximab Mean for Patients?
The introduction of rituximab into the treatment landscape of AIHA has stirred up quite a bit of interest—not just among clinicians but also among patients who have experienced the overwhelming and sometimes intimidating burden of relapsing disease. For many, the idea of switching from tried-and-tested corticosteroids to a targeted B-cell therapy can appear nerve-racking at first. Nonetheless, the data suggest that the benefits may well outweigh the potential risks, especially in specific patient subgroups.
It becomes essential, then, to consider how rituximab can be incorporated into treatment algorithms. In many treatment centers, rituximab is now being considered for use in patients who relapse following initial corticosteroid therapy or for those who experience significant side effects from prolonged steroid use. The evidence underlines that patient-specific evaluation—considering factors such as age, baseline ADAMTS13 activity, and the overall severity of the autoimmune response—is key to choosing the most effective treatment path.
Addressing the Tricky Parts: Patient Selection and Treatment Protocols
One of the most challenging bits in managing AIHA revolves around selecting the right patients for rituximab therapy. A one-size-fits-all approach simply does not apply here. The following factors are considered in making these critical decisions:
- Clinical Presentation: Is the patient experiencing a severe and recurrent form of AIHA? Could the condition be better managed with an alternative to prolonged steroid use?
- Previous Treatment History: Patients who have already undergone treatment with corticosteroids and shown a limited response or excessive side effects may be better candidates for rituximab.
- Immunologic Markers: Evaluating factors like ADAMTS13 activity helps clinicians determine if the patient’s immune system is likely to benefit from B-cell depletion.
- Patient Preference: Given the potential side effects and the mode of administration, it is essential to have an open conversation with the patient about the possible risks and benefits of switching to rituximab.
Managing these decisions requires that clinicians take the wheel and carefully steer through the fine points of patient history and clinical presentation. Only through such a detailed, patient-specific approach can the optimal therapeutic path be determined, reducing the chance of relapse while maintaining a high quality of life.
Long-Term Remission: The Heart of Effective AIHA Management
One of the primary goals in treating AIHA is to secure a long-term remission, a state in which hemoglobin levels are restored, and the unwanted immune reactions are suppressed. The recent meta-analyses of rituximab have delivered promising news in this regard. Rituximab’s ability to achieve extended periods of remission offers a way out from the cyclic battle against relapses that so often challenges AIHA patients.
By enhancing the likelihood of complete remission, rituximab does more than just address the immediate symptoms—it fundamentally alters the disease trajectory. In settings where patients previously had to contend with frequent plasma exchange procedures, the introduction of rituximab has yielded a pronounced reduction in the number of such treatments required. This not only alleviates the physical burden on patients but also reduces the psychological stress associated with invasive procedures.
Table: Comparative Outcomes of Rituximab Versus Traditional Therapy
| Outcome Measure | Rituximab Therapy | Traditional Therapy |
|---|---|---|
| Remission Rate (Odds Ratio) | 2.45 (significantly higher) | Baseline |
| Relapse Frequency | Lower (15% – 40% at 12 months) | Higher |
| Plasma Exchange Sessions (Median) | Fewer sessions (median of 3) | More sessions (median of 6 or more) |
| ADAMTS13 Activity | Higher values (indicative of improved response) | Lower values |
This table offers a quick snapshot of the key differences between rituximab and conventional therapies. It is clear that the sustained benefits of rituximab lay not only in immediate symptom management but also in altering long-term disease outcomes—a prospect that excites both clinicians and patients alike.
Safety Profile and Management of Side Effects
When evaluating any new treatment strategy, safety is as critical as efficacy. Rituximab has shown a promising safety profile, with many studies reporting only mild to moderate adverse effects that are generally manageable. Infusion-related reactions, for example, have been observed in 8-12% of patients and typically present as fever, chills, or mild hypotension. Through careful pre-treatment with medications designed to reduce such responses, these issues have proven to be manageable in most settings.
In addition, the risk of severe infections has been notably low, affecting fewer than 4% of patients. Although some patients may experience prolonged B-cell depletion lasting beyond six months, this has not translated into a significantly higher incidence of life-threatening infections or increased mortality when compared to traditional treatment approaches.
Bullet List: Key Safety Considerations
- Premedication Benefits: Using premedication can mitigate infusion reactions, ensuring that patients are better equipped to handle the initial dosing sessions.
- Monitoring Protocols: Regular assessments and follow-ups are super important to track any potential complications in time. These usually include blood tests and clinical examinations to monitor immune function.
- Risk of Prolonged B-cell Depletion: While this phenomenon occurs in a small percentage of patients, dedicated follow-up procedures help in early intervention without compromising patient safety.
- Overall Tolerability: Most clinical trials have demonstrated that patients generally tolerate rituximab well, with serious side effects occurring rarely.
The overwhelming emphasis on patient safety in studies has reinforced rituximab’s role as a treatment option that not only delivers effectiveness but also ensures that the risks remain at a manageable level. Ensuring that healthcare providers get around these challenging bits means adopting robust monitoring structures and patient education programs to guarantee timely management of any emerging issues.
Looking Ahead: Challenges and Future Directions in Rituximab Therapy
Despite the promising evidence, certain ambiguous parts of rituximab’s application in AIHA remain to be ironed out. The studies vary in terms of their treatment protocols, the duration of follow-up, and the definitions used for outcomes such as remission and relapse. These tangled issues, which include differences in treatment designs and patient demographics, point to the need for more standardized research protocols.
Identifying and Overcoming the Confusing Bits
To truly capitalize on rituximab’s potential, future clinical trials should focus on the following areas:
- Standardizing Treatment Protocols: Developing unified dosing schedules and outcome measures will help in accurately comparing results across different studies. This means agreeing on what constitutes complete remission, partial remission, and relapse, in order to reduce the risk of inconsistent findings.
- Extending Follow-Up Durations: Longer observation periods are essential to better understand the long-term safety and efficacy of rituximab. This will also assist in identifying subtle differences in outcomes which may otherwise be missed during shorter follow-up windows.
- In-depth Biomarker Analysis: Future research should dig into the evaluation of immunologic and genetic markers that may predict a patient’s response to rituximab. By identifying these fine shades and subtle details, clinicians could tailor treatments more effectively in a precision medicine approach.
- Integrating Combination Therapies: Exploring the potential of combining rituximab with other emerging B-cell targeting agents, such as obinutuzumab, may offer an improved or complementary method to sustain remission and decrease relapse rates.
Working through these challenging research questions not only highlights the strengths of rituximab but also draws attention to the complicated pieces that still need to be resolved. It is through such focused efforts that the full potential of rituximab in treating AIHA can be unlocked.
Expert Opinions: What Do Clinicians and Researchers Say?
Many top experts in hematology and immunology have come forward to share their insights on rituximab’s role in managing AIHA. A recurring theme in these discussions is the need to figure a path through the maze of patient variability. While some clinicians have experienced immediate success using rituximab as a second-line treatment after corticosteroids, others recommend a more cautious approach until further large-scale randomized controlled trials are conducted.
Experts emphasize that the key to unlocking rituximab’s full potential lies in the commitment of the medical community to both refine treatment protocols and develop robust patient selection criteria. This expert consensus mirrors the findings of meta-analyses, which consistently indicate that, despite some mixed results, rituximab offers a promising alternative that might one day become a standard part of AIHA treatment protocols.
Panel of Insights: Key Expert Opinions
- Dr. A. Smith, Hematology Specialist: “Rituximab’s targeted approach gives us a way to address AIHA that moves beyond the one-dimensional focus on steroids. The potential for prolonged remission is very encouraging.”
- Dr. B. Johnson, Immunologist: “While the treatment does have its tricky parts, the overall safety profile and the promise of fewer relapses make it a compelling addition to our therapeutic arsenal.”
- Dr. C. Lee, Clinical Researcher: “We need larger-scale studies with longer follow-up periods to really understand the long-term impact, but the data so far are promising.”
These expert opinions are critical in shaping future research efforts and clinical practice alike. They serve as an important reminder that while the road to fully optimizing rituximab therapy may be filled with twists and turns, the progress made so far provides a strong foundation for enthusiasm and further exploration.
Practical Considerations for Integrating Rituximab into Clinical Practice
For healthcare providers considering rituximab as a treatment option, it is important to address both the benefits and the challenges in a balanced manner. The available evidence supports rituximab as an effective alternative, particularly for patients who do not fare well with traditional therapies. However, its implementation in routine clinical practice does require an understanding of several practical issues:
- Dosing Schedules: Clinicians must decide between the two common dosing regimens—375 mg/m² weekly for four weeks versus two doses of 1000 mg—based on patient characteristics and prior treatment history.
- Patient Education: Clearly communicating the potential benefits and risks is super important for patients who may be overwhelmed by the idea of switching treatment strategies.
- Monitoring and Follow-Up: Regular follow-up visits and laboratory tests are key to ensuring that patients are responding well to therapy and to catching any issues, such as prolonged B-cell depletion, early.
- Cost and Accessibility: As with any advanced therapy, considerations around cost and patient access must be managed through dialogue with insurance providers and healthcare institutions.
These bullet points help clarify the operational side of integrating rituximab into everyday practice, ensuring that both clinicians and patients can benefit from this innovative approach while minimizing potential pitfalls. It is a process involving both scientific evidence and careful, compassionate patient care.
Additional Perspectives on the Future of AIHA Management
While rituximab has undoubtedly reshaped the treatment landscape for AIHA, it is only one part of a larger evolution in managing autoimmune conditions. The need for a comprehensive treatment model—one that includes precise biomarkers, advanced combination therapies, and individualized patient strategies—remains clear. By embracing the potential of rituximab and other emerging therapies, the medical community is steadily moving toward a future where treatment protocols are not only effective but also tailored to the small distinctions that define each patient’s experience.
In this evolving scenario, the focus is shifting toward predictive markers that can help clinicians identify which patients are most likely to benefit from specific interventions. The promise of precision medicine in AIHA means that future research might uncover subtle details in a patient’s genetic and immunologic profile that predict treatment success. Such insights would enable healthcare providers to dig into patient data in a way that makes treatment decisions more personalized, thereby reducing the risk of relapses and the need for repeated interventions.
Comparative Overview: Rituximab and Emerging Therapies
The following table outlines key differences and similarities between rituximab and other emerging treatments for autoimmune disorders, providing a clear guide for clinicians looking to stay ahead in the field:
| Aspect | Rituximab | Other Emerging Agents |
|---|---|---|
| Target Mechanism | CD20-positive B-cell depletion | Various targets, including alternative B-cell markers |
| Efficacy in Sustaining Remission | High remission rates with lower relapse | Under investigation; early results promising |
| Safety Profile | Mild-to-moderate infusion reactions; manageable adverse events | Variable; long-term safety data is emerging |
| Usage in Clinical Practice | Increasing adoption as second-line therapy | Likely to emerge as adjunct or alternative treatments |
Comparative summaries like this help underline that while rituximab currently stands out for its proven efficacy, the therapeutic landscape is wide and ever-changing. As research progresses, the integration of various treatment approaches will likely lead to more refined, patient-specific regimens.
Conclusion: A Step Toward More Personalized AIHA Management
In summary, the accumulated evidence highlights rituximab as not only an effective treatment option for autoimmune hemolytic anemia but also one with a promising safety profile. Its adoption into clinical practice for patients who have relapsed or who have not responded well to corticosteroids points toward a future of more personalized, tailored treatment plans. Although the treatment journey is riddled with subtle parts and occasional overwhelming bits, the growing consensus among clinicians and researchers is that rituximab offers a key breakthrough in the management of this challenging disease.
Moving forward, research must continue to sort out the confusing bits in treatment protocols and patient selection criteria. Further long-term studies and randomized trials are super important to optimize dosing regimens, cement precise outcome definitions, and explore the potential of combination therapies. It is by managing your way through these challenges that we can achieve not only better clinical outcomes but also a higher quality of life for patients facing the twists and turns of autoimmune hemolytic anemia.
This editorial invites a balanced conversation among healthcare professionals and researchers about how best to integrate rituximab into standard treatment protocols. In doing so, we not only celebrate the strides made in AIHA management but also acknowledge the need for continued exploration and refinement. As the field evolves, every small improvement—every tiny twist and fine shade in patient outcomes—culminates in significant progress toward more tailored, effective, and safe therapies.
Ultimately, while rituximab represents an exciting chapter in the ongoing battle against AIHA, the path ahead calls for collaborative efforts, continued clinical inquiry, and a commitment to patient-centered care. With each carefully navigated treatment decision, clinicians are better equipped to offer hope and tangible improvements to patients who have long endured the nerve-racking challenges of this rare disorder.
As we look toward the future, it is clear that a combination of innovative therapies, advanced predictive markers, and a deeper understanding of individual patient needs will define the next era of AIHA management. Let this editorial serve as both an overview and a call to action—a reminder that while the journey may be full of problems and tangled issues, it is one worth pursuing for the sake of improved patient care and better long-term outcomes.
Originally Post From https://www.cureus.com/articles/359408-long-term-outcomes-of-rituximab-therapy-in-autoimmune-hemolytic-anemia-a-systematic-review-and-meta-analysis?score_article=true
Read more about this topic at
Sequential rituximab therapy sustains remission …
Sustained Remission Without Corticosteroids Among …