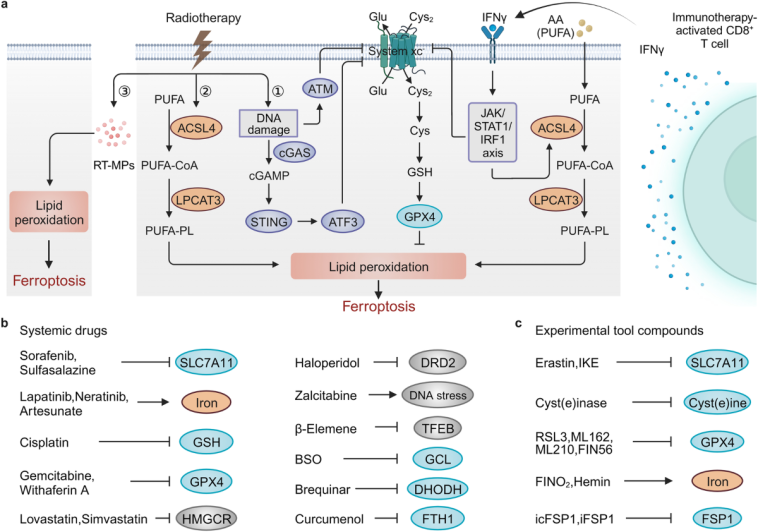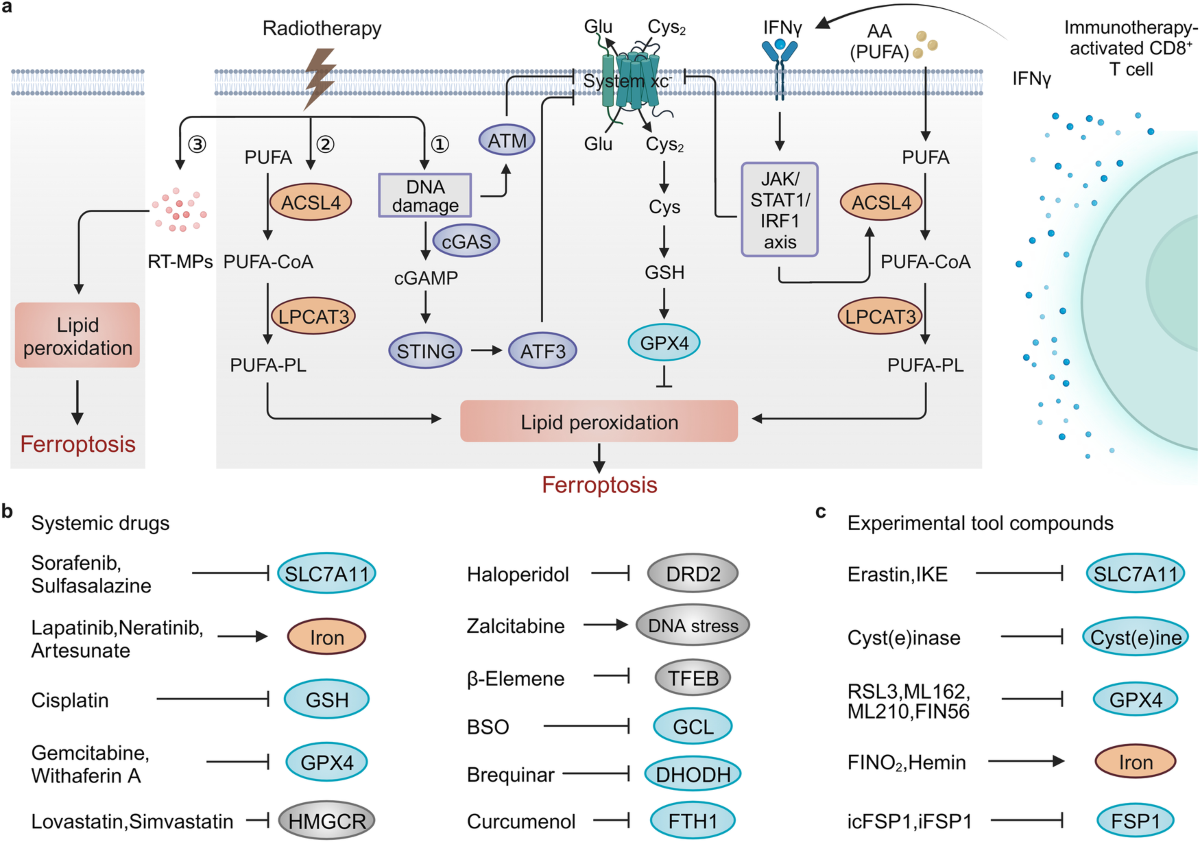
Ferroptosis Therapies in Cancer: An Evolving Frontier
Cancer research is steadily unearthing new ways to tackle malignant cells, and one of the most exciting developments in recent years is the investigation of ferroptosis—a unique, nonapoptotic form of cell death defined by lethal membrane lipid peroxidation. As we figure a path through this emerging field, the interplay between iron metabolism, lipid peroxidation, and the immune system promises both opportunity and challenging bits needing further exploration in the journey to develop new anticancer therapies.
In this opinion editorial, we take a closer look at the hidden complexities of ferroptosis, its promise as an anticancer strategy, and the many twists and turns that researchers must overcome to convert bench-side promise into useful clinical tools. Along the way, we’ll dive into the fine points of its mechanism, discuss potential pitfalls in translation, and share perspectives on how the oncology community can better harness this pathway.
Understanding the Lethal Lipid Peroxidation Pathway
At its core, ferroptosis hinges on a simple yet deadly concept: the destruction of cellular membranes through iron-dependent lipid peroxidation. Unlike apoptosis or necrosis, this mode of cell death bypasses many of the usual cellular checkpoints, making it an enticing target against cancer cells that have developed resistance to other therapies. However, getting into the nitty-gritty of the ferroptosis mechanism reveals a number of tangled issues and tricky parts that demand our careful attention.
The Mechanism Uncovered: Iron, Lipids, and Beyond
During ferroptosis, cells experience an imbalance in their oxidative defense systems causing an uncontrolled buildup of reactive oxygen species (ROS) that attack polyunsaturated fatty acids in the cell membrane. This breakdown leads to a cascade of events resulting in cell death. Key regulators such as glutathione peroxidase 4 (GPX4) and the cystine–glutamate antiporter (xCT) play a central role in keeping iron and lipid oxidation in check.
Below is a simplified outline summarizing the mechanism:
- Iron Overload: Excess iron catalyzes the formation of ROS and serves as a starting point for lipid peroxidation.
- Lipid Peroxidation: Reactive oxygen species attack membrane lipids, causing structural damage and cell lysis.
- Antioxidant Defense Systems: Enzymes like GPX4 help mitigate oxidative damage by reducing lipid hydroperoxides. Disruption of this defense is key to inducing ferroptosis.
- Cell Death Execution: Once the chain reaction is set in motion, the breakdown of cellular membranes results in cell death independent of the classical apoptotic pathway.
Understanding these steps in precise detail is critical, yet the process is not linear. There are several fine shades and subtle details that add layers of complexity. For instance, the balance of intracellular iron levels is affected by metabolic pathways, cell cycle status, and even the tumor microenvironment, rendering the process both fascinating and, at times, overwhelming.
Translating Laboratory Discoveries to Real-World Therapies
While laboratory studies on ferroptosis have uncovered promising targets, translating these findings into clinical strategies is full of confusing bits and nerve-racking challenges. The journey from understanding the basic science to designing treatments that can selectively trigger ferroptosis in tumors involves navigating a maze of regulatory, biological, and technical hurdles.
Challenges in Drug Development and Clinical Testing
One of the most intimidating aspects of ferroptosis research is designing therapies that reliably induce this form of cell death in cancer cells without inflicting unacceptable damage to normal tissue. Several problems contribute to this difficulty:
- Off-Target Effects: Many ferroptosis inducers can affect healthy cells, leading to side effects due to the non-specific nature of oxidative stress.
- Resistance Mechanisms: Cancer cells are adept at activating compensatory antioxidant pathways, thereby dampening the intended effect of ferroptosis inducers.
- Delivery Challenges: Achieving adequate drug concentration in tumors while avoiding systemic toxicity is a tricky part of the therapeutic equation.
- Biomarker Identification: The success of ferroptosis-based therapy may rely on detecting subtle details of lipid oxidation and iron metabolism, but standard biomarkers remain under development.
In many ways, the challenge here is like finding your way through a crowded, twisting maze where each turn may reveal yet another obstacle or opportunity. Successfully addressing these points is key to future breakthroughs.
Case Studies and Experimental Models
Recent experiments have demonstrated that cell lines and animal models with high intracellular iron content or defective antioxidant defenses exhibit increased sensitivity to ferroptosis inducers. Researchers have noted that coupling ferroptosis inducers with existing chemotherapies or immunotherapies may provide synergy, potentially overcoming drug resistance. However, it’s worth noting that these results are often loaded with issues related to varying experimental conditions and complex cell-environment interactions.
Table 1 shows an overview of some experimental ferroptosis inducers, their targets, and observed responses in preclinical models:
| Compound | Primary Target | Model Outcome |
|---|---|---|
| Erastin | xCT (cystine transporter) | Increased lipid peroxidation; cell death in resistant cancer types |
| RSL3 | GPX4 inhibition | Rapid induction of ferroptosis in certain tumor cell models |
| FIN56 | GPX4 degradation | Enhanced cell death when combined with metabolic inhibitors |
This table exemplifies how different approaches can target various facets of the ferroptosis pathway, offering multiple angles for future drug development.
Iron, Lipids, and the Tumor Microenvironment: The Hidden Complexities
The role of iron and lipids in ferroptosis is not just a simple on-off switch but a finely tuned interplay involving multiple regulatory systems. The tumor microenvironment (TME) adds another layer of subtle details that make the application of ferroptosis-based therapies especially intriguing.
Considering the Tumor Microenvironment
Tumor cells often reside in a niche that is both hypoxic and immune-suppressed, where metabolic adaptation is critical. In these settings, cancer cells may accumulate iron or alter lipid composition as a survival tactic, rendering them more or less sensitive to ferroptotic triggers. For instance, immune cells in the TME could be influenced by the lipid signals released from dying tumor cells, further modulating antitumor immune responses.
Key elements of the microenvironment include:
- Immune Cell Interactions: The exchange of signals between cancer cells and immune cells, like dendritic cells and T cells, may either trigger or inhibit ferroptosis. These interactions are replete with small distinctions that researchers must navigate carefully.
- Oxygen and Nutrient Levels: Areas within tumors that suffer from nutrient deprivation or limited oxygen can alter the expression of ferroptosis-regulating proteins.
- Extracellular Matrix Components: The scaffolding around cells can influence drug delivery and modulate access to vital nutrients, thereby affecting the induction of ferroptosis.
Understanding these factors is super important as they may dictate the success or failure of targeted ferroptosis therapies. Researchers must now steer through these variable conditions to design strategies that are effective across different tumor types.
Integrating Metabolic Vulnerabilities
Cancer cells typically reprogram their metabolism to support rapid proliferation. This metabolic reprogramming can introduce vulnerabilities that ferroptosis inducers might exploit. However, disentangling these relationships involves finding your way through a maze of tangled metabolic pathways.
For example, a subset of cancer cells exhibits:
- A dependency on cysteine uptake to maintain glutathione levels
- Enhanced lipid synthesis creating an accumulation of polyunsaturated fatty acids
- An altered iron metabolism that predisposes them to oxidative stress
Targeting these aspects requires careful design and evaluation, as well as an understanding of the subtle interplay between various metabolic circuits. This dual focus on metabolism and cell death pathways could ultimately open up new therapeutic windows.
Immunological Implications: Harnessing the Body’s Own Defenses
One of the most promising angles of ferroptosis-based therapy is its potential to work hand-in-hand with the body’s immune system. As cancer cells undergo ferroptosis, they release signals that can stimulate an antitumor immune response, potentially enhancing the efficacy of immunotherapies.
Linking Ferroptosis and Immune Activation
Recent studies suggest that the lipid peroxidation products generated during ferroptosis serve as damage-associated molecular patterns (DAMPs) that alert the immune system. When immune cells such as T cells and macrophages detect these signals, they can ramp up their antitumor efforts. Yet, this process is replete with little twists—where the same signals, if unregulated, might also contribute to a pro-tumor environment by triggering immune suppression.
Researchers are now trying to figure a path that maximizes the beneficial immune-stimulating effects while minimizing the risk of inadvertently dampening immune function. This balancing act is one of the key challenges in integrating ferroptosis with immunotherapy strategies.
Combining Ferroptosis Inducers with Immunotherapy
One emerging strategy involves the simultaneous use of ferroptosis inducers with checkpoint inhibitors, a type of immunotherapy designed to unleash T cells on tumor cells. Early experimental evidence indicates that:
- Ferroptotic cell death in tumor cells can enhance antigen presentation
- Combined treatment might overcome resistance mechanisms seen with single-agent therapies
- Immune cells may become better equipped to recognize and destroy tumor cells after exposure to ferroptosis mediators
This combination approach is gaining momentum, although clinical trials remain in the early phases. Careful dissection of the fine shades between pro- and anti-tumor immune effects will be critical for future therapeutic success.
Safety Considerations and Off-Target Effects
As with any new therapeutic approach, the safety profile of ferroptosis inducers is a paramount concern. Although the idea of selectively triggering cell death in cancer cells is appealing, the potential for off-target effects in normal tissues introduces a number of complicated pieces that researchers must address.
Risks of Unwanted Oxidative Damage
Ferroptosis involves driving cells to a state of oxidative stress that can, in theory, affect neighboring healthy cells if not properly contained. To counter this, researchers are exploring methods such as:
- Targeted Delivery Systems: Nanoparticles and antibody-drug conjugates that direct inducers specifically to cancer cells.
- Prodrug Strategies: Designing ferroptosis inducers that are activated only in the unique tumor microenvironment.
- Combination Therapies: Using multiple agents at lower doses to reduce the risk of systemic toxicity while still effectively inducing cell death in tumors.
Each of these strategies represents a move toward making ferroptosis-based treatments both safer and more effective. The process is replete with potential pitfalls, making it essential for researchers to get into the fine details of both drug design and delivery.
Monitoring and Biomarker Development
A further challenge is the ability to monitor ferroptosis in real time and assess the efficacy of treatment. Developing reliable biomarkers that indicate when cells are undergoing ferroptosis is critical for tailoring therapies to individual patients. This involves:
- Identifying specific lipid oxidation products that correlate strongly with ferroptosis
- Developing imaging techniques to detect these oxidative markers in tumors
- Creating blood-based biomarkers that can serve as early indicators of therapeutic response
Although these efforts are still in the early stages, progress in this area will be super important for guiding clinical decisions and ensuring that treatments remain both effective and safe.
Learning from Preclinical Models and Early Clinical Trials
Several preclinical studies and early-phase clinical trials are beginning to shed light on the feasibility of using ferroptosis inducers in cancer therapy. While the road ahead is loaded with problems, these studies are invaluable as they help us figure a path through the challenging terrain.
Highlights from Preclinical Evidence
A range of studies have demonstrated that ferroptosis can be effectively triggered in various cancer cell types, including:
- Melanoma, where lymphatic signals have an unexpected role in protecting metastasizing cells from ferroptosis
- Pancreatic tumors, where cysteine depletion has been shown to sensitize cells to oxidative stress
- Breast cancer and certain aggressive lymphomas that show a dependency on glutathione metabolism and iron homeostasis
These findings have catalyzed interest in combining ferroptosis inducers with other targeted modalities, such as inhibitors of glutathione synthesis or inducers of immune cell activation.
Early Clinical Trials and Future Directions
Clinical trials exploring ferroptosis-based interventions remain in the early stages, and their design is fraught with twists and turns. Some pilot studies have begun to examine the safety of these agents, often in combination with established treatments. The key issues under investigation include:
- The optimal dosing regimen that maximizes tumor cell death while minimizing harm to healthy tissues
- Patient selection criteria based on biomarkers of iron metabolism and oxidative stress
- Synergistic effects when combining ferroptosis inducers with chemotherapy, radiotherapy, or immunotherapy
Although it is too early to definitively assess the clinical potential of ferroptosis therapies, these early endeavors set the stage for more extensive trials that could revolutionize cancer treatment.
Overcoming the Twists and Turns: Research and Development Strategies
To fully harness the promise of ferroptosis in cancer therapy, stakeholders—from academic investigators and pharmaceutical companies to regulatory bodies—must work together to sort out the tangled issues that have so far hindered rapid progression toward clinical use.
Investment in Basic Research and Biomarker Discovery
A robust understanding of ferroptosis at the molecular level remains a prerequisite for developing effective therapies. Increased funding for basic research is essential to elucidate the subtle parts of the ferroptotic pathway, refine potential targets, and ultimately translate these findings into the clinic. Key research priorities include:
- Decoding the iron and lipid metabolism in various tumor types
- Mapping out the cross-talk between ferroptosis and other cell death pathways
- Developing early-warning biomarkers for treatment response
By prioritising these areas, the scientific community can begin to unravel the complicated pieces that characterize ferroptosis, thereby making future therapies more robust and predictable.
Cross-Disciplinary Collaboration: Bringing Together Diverse Expertise
Ferroptosis research is inherently multidisciplinary. It requires close collaboration among biochemists, oncologists, immunologists, and medicinal chemists, as well as input from experts in nanoparticle drug delivery and bioinformatics. This cross-disciplinary cooperation is key to managing your way through scientific hurdles, ensuring that each perspective—be it biochemical, clinical, or technological—informs the overall strategy.
Collaborative initiatives could include:
- Joint research projects across academic and industry laboratories
- Workshops and conferences dedicated to ferroptosis and metabolic vulnerabilities in cancer
- Public-private partnerships focused on accelerating the development of novel ferroptosis inducers
Working together in this way will help streamline efforts, reduce redundant work, and ensure that breakthroughs in one area rapidly inform efforts in another.
Embracing a Future of Personalized Ferroptosis Therapies
Personalized medicine is quickly becoming a key trend in oncology, and ferroptosis therapies could be a notable addition to this personalized toolkit. By tailoring approaches to the specific molecular makeup of a patient’s tumor, clinicians can improve outcomes and reduce side effects.
Tailoring Treatments to Tumor Metabolism
Cancer cells differ widely in the way they regulate iron, lipid synthesis, and antioxidant defenses. This heterogeneity means that a “one-size-fits-all” approach to ferroptosis is unlikely to succeed. Instead, treatments must be designed to target the unique metabolic profile of each tumor. For example:
- Patients with tumors that exhibit high iron uptake might benefit best from agents that exacerbate iron-mediated ROS production.
- Those with defects in the GPX4 pathway could be more sensitive to compounds that specifically inactivate this enzyme.
- Combination therapies that address both metabolism and immune evasion could be tailored for patients with more aggressive or resistant forms of cancer.
Moving toward personalized ferroptosis therapy will require the development and validation of predictive biomarkers, which will guide clinicians in selecting the most appropriate therapeutic options for each individual.
Integrating Advanced Diagnostics and Imaging
Advances in imaging and diagnostics provide new opportunities to monitor ferroptosis in real time. Techniques such as mass spectrometry-based lipid profiling, advanced MRI protocols, and molecular imaging markers are being developed to help assess the extent of lipid peroxidation and oxidative damage in tumors.
These tools offer a promising future for:
- Real-time monitoring of treatment response during therapy
- Early detection of adverse off-target effects, ensuring patient safety
- Adjustment of therapeutic strategies based on dynamic changes in the tumor microenvironment
Together, these innovations could help clinicians figure a path to more precisely control drug dosing and scheduling, thereby enhancing the overall effectiveness of ferroptosis-based interventions.
Future Perspectives and Concluding Thoughts
The journey toward integrating ferroptosis therapies into cancer treatment is an adventure full of exciting opportunities and challenging bits. With every breakthrough in our understanding of how iron and lipid metabolism drive cell death, the promise of new, more effective treatments for resistant cancers grows stronger.
There remain many twists and turns on the path forward—ranging from drug delivery issues and off-target risks to the daunting task of developing robust biomarkers for clinical use. Yet, the concerted efforts of researchers across disciplines have already begun to chip away at these barriers.
Key Takeaways for Future Research
In summary, here are some of the essential points to keep in mind as the field develops:
- Understanding Mechanisms: A deep appreciation of the fine points of ferroptosis is crucial for developing effective therapies.
- Overcoming Delivery Challenges: Innovative delivery mechanisms such as targeted nanoparticles and prodrug strategies will be key to achieving clinical success.
- Safety and Biomarkers: Monitoring for off-target effects using advanced imaging and reliable biomarkers is a must-have step in therapy development.
- Immunotherapy Synergy: Combining ferroptosis inducers with immunotherapies holds great promise, provided that the subtle details of immune activation are carefully managed.
- Personalized Approaches: Tailoring treatments to individual tumor profiles is likely to maximize efficacy while minimizing side effects.
These points emphasize that while the path toward effective ferroptosis therapies is lined with challenges, the potential rewards—improved outcomes for patients facing some of the most resistant forms of cancer—are well worth the effort.
A Call to Action for the Scientific Community
The prospect of harnessing ferroptosis in the fight against cancer is not just an academic exercise; it is a call to arms for researchers, clinicians, and industry stakeholders to work together in addressing the tricky parts, tangled issues, and overwhelming challenges that define this field. With interdisciplinary collaboration, strategic investment in fundamental research, and steadfast attention to patient safety, we can look forward to a future where ferroptosis-based therapies become a standard part of the oncology armamentarium.
In closing, the promise of ferroptosis in cancer therapy stands as a beacon of hope amid the many complications of tumor biology. The pace of discovery over the past decade is encouraging, yet much work remains. As we continue to explore these hidden complexities and optimize treatment strategies, one thing is clear: the fight against cancer is entering a promising new era, one where understanding and manipulating cell death could save countless lives.
Looking Ahead: The Road to Clinical Integration
The future of cancer therapy is a dynamic landscape where advances in understanding metabolic reprogramming and cell death will inevitably lead to more effective treatments. Ferroptosis, with its myriad layers of regulation and potential for selective targeting, illustrates just how complex and promising this evolving field can be.
Key developments to watch include:
- Clinical Trials: Ongoing and upcoming studies that will rigorously test the safety and efficacy of ferroptosis inducers in diversified patient populations.
- Combination Regimens: Innovative protocols combining ferroptosis inducers with established therapies, such as chemotherapy, radiotherapy, and immunotherapy, to overcome resistant cancer phenotypes.
- Technological Advances: Improvements in molecular imaging and diagnostic assays that allow for an accurate readout of ferroptosis in tumors.
- Personalized Medicine: The development of patient-specific therapeutic regimens based on the metabolic profile and ferroptosis sensitivity of their tumors.
Integrating these developments will require a careful review of both preclinical evidence and early clinical results, ensuring that the transition from bench to bedside is as smooth as possible. In doing so, scientists and clinicians alike must be prepared to take on the nerve-racking, twist-filled journey ahead, one step at a time.
Overcoming Regulatory and Logistical Hurdles
One of the more intimidating aspects of developing ferroptosis-based therapies involves the maze of regulatory and logistical hurdles that accompany any new drug development process. Bringing a new therapeutic concept from the lab to the clinic demands rigorous testing, robust safety data, and compliance with international standards—all of which take time and considerable effort.
Key considerations include:
- Regulatory Approval: Navigating the approval process requires comprehensive data on drug safety, pharmacokinetics, and efficacy. Agencies like the FDA and EMA will demand robust evidence from preclinical models and human trials.
- Intellectual Property: Securing patents and protecting innovations in ferroptosis inducers is essential for encouraging investment and fostering further research.
- Manufacturing and Scale-Up: Developing scalable synthesis protocols for these compounds that maintain their stability and efficacy is no small feat.
- Cost and Accessibility: Ensuring that new ferroptosis-targeted therapies are accessible and affordable to patients worldwide is a long-term goal that must be addressed early in the development process.
Each of these aspects represents a small—but crucial—turn in the road toward integrating ferroptosis therapy into everyday clinical practice.
Conclusion: The Promise and the Challenge
As we stand at the brink of a potential revolution in cancer therapy, ferroptosis represents both a beacon of hope and a reminder of the many complicated pieces that still need to be solved. With its unique mechanism of inducing cell death, promising experimental results, and potential to synergize with immunotherapy, ferroptosis is undeniably an area where great breakthroughs might lie.
However, this journey is not without its challenges. From understanding the fine details of lipid peroxidation to designing targeted delivery systems, from overcoming off-target effects to integrating personalized medicine approaches—the hurdles are many, and the road ahead is peppered with nerve-racking twists and turns.
Nevertheless, the continued collaborative efforts of scientists, clinicians, and industry stakeholders are paving the way for innovative therapies. By investing in deeper basic research; developing robust diagnostic and monitoring tools; and rigorously testing combination strategies in clinical trials, we can look forward to a future where ferroptosis not only demystifies the process of cancer cell death but also transforms it into a practical, life-saving weapon.
In the end, the story of ferroptosis therapies in cancer is a testament to the resilience and ingenuity of the research community. It is a reminder that even the most complicated challenges—filled with confusing bits, intimidating hurdles, and tangled issues—can eventually be overcome when we work together with focused determination and creative problem solving.
As we embrace this promising, yet challenging, new frontier, it is clear that the road to effective ferroptosis therapies will require both innovation and perseverance. With every new discovery, we are one step closer to unleashing the full potential of this exciting strategy in the battle against cancer—and that is a journey worth taking.
Originally Post From https://www.nature.com/articles/s43018-025-01037-7
Read more about this topic at
Prospects for ferroptosis therapies in cancer
Harnessing ferroptosis for precision oncology: challenges and …