Examining First-line Chemotherapy Options in Advanced Biliary Tract Cancer
The treatment of advanced biliary tract cancer is a subject full of complicated pieces and many tricky parts that touch on both modern medicine and alternative approaches. In our discussion today, we get into the details of a recent phase II clinical trial comparing two first-line regimens: nab-paclitaxel plus cisplatin (NC) versus the more established gemcitabine plus cisplatin (GC). This editorial provides an opinion-based look at the study’s findings, offering insights on progression-free survival, overall survival, safety profiles, and the potential for future therapeutic options. We will use tables, bullet lists, and subheadings with long-tail keywords to help figure a path through these dense clinical findings.
Understanding Advanced Biliary Tract Cancer and Its Treatment Challenges
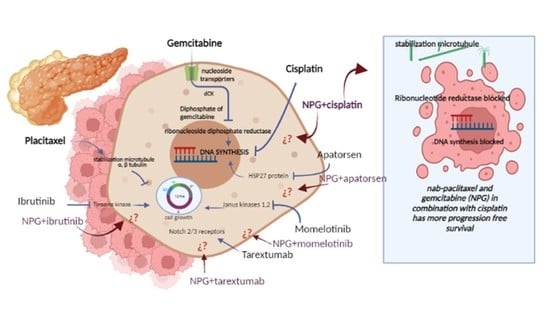
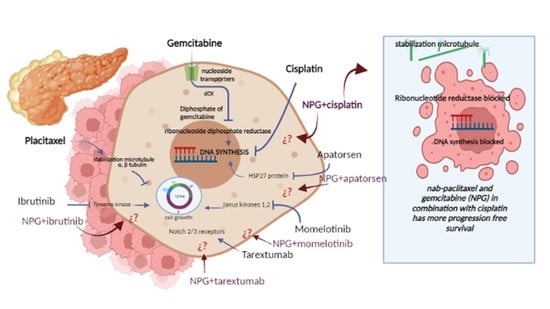
Advanced biliary tract cancer, which covers cholangiocarcinoma and gallbladder cancer, presents many nerve-racking challenges, particularly because the disease is often diagnosed late. With limited surgical options available to most patients, systemic chemotherapy becomes the cornerstone treatment. Conventional therapies, like gemcitabine combined with cisplatin, have been in use for years. Yet, these regimens carry their own set of tangled issues, including substantial toxicity and only modest improvements in survival rates.
Clinicians and researchers alike have been looking for ways to improve outcomes by exploring alternative combinations while trying to avoid the intimidating side effects typically associated with aggressive chemotherapy. This editorial examines how an alternative regimen, which pairs nab-paclitaxel with cisplatin, might offer a similar level of efficacy with a potentially better side effect profile.
Comparing Nab-paclitaxel Plus Cisplatin and Gemcitabine Plus Cisplatin
A multicentre, randomized, phase II trial recently compared these two chemotherapy regimens in patients with advanced biliary tract cancer. While both groups shared similar overall survival results, notable differences emerged in progression-free survival and safety profiles. Before we get into the study details, it is valuable to outline the key points in bullet form:
- Study Population: 75 patients diagnosed with unresectable or advanced-stage biliary tract cancer.
- Regimens Tested: The experimental NC group (nab-paclitaxel + cisplatin) versus the control GC group (gemcitabine + cisplatin).
- Primary Endpoint: Progression-free survival (PFS).
- Secondary Endpoints: Overall survival (OS), objective response rate (ORR), and chemotherapy-related adverse events.
- Study Duration: Patients were followed for a median period of approximately 11 months.
This design allowed the investigators to dig into the fine points of efficacy while keeping an eye on key safety concerns, an approach that could guide future larger-scale studies.
Key Study Outcomes: A Closer Look at Progression-Free and Overall Survival
One of the standout aspects of this trial was the difference in the median progression-free survival between the two regimens. The NC group exhibited a median PFS of 7.8 months compared to 7.0 months in the GC group. Although the difference might seem subtle, the statistical significance (with a hazard ratio of roughly 0.514) indicates that the experimental regimen could offer clinically meaningful benefits in delaying disease progression.
It is important to note, however, that the overall survival, which serves as a more holistic measure of patient benefit, was nearly identical between both groups. The NC group achieved a median OS of 12.4 months versus 12.1 months in the GC group. Such results raise the question of whether nab-paclitaxel-based regimens might serve as a suitable alternative for patients specifically concerned about the side effects associated with gemcitabine.
In practical terms, the increased PFS may offer patients a longer period with acceptable quality of life before experiencing significant disease advancement, an aspect that is often just as critical as overall survival in a clinical context.
Safety and Tolerability Profiles: A Tabular Comparison
The safety profile is one area where this trial provided illuminating insights. When it came to adverse events, both regimens were generally manageable. However, there were key differences in the types of side effects that each group experienced. The table below highlights the main differences observed in toxicity:
| Adverse Event Category | GC Regimen (Gemcitabine + Cisplatin) | NC Regimen (Nab-paclitaxel + Cisplatin) |
|---|---|---|
| Hematologic Toxicity (Thrombocytopenia) | Higher incidence (50% experienced reduction in platelet count) | Lower incidence (32.4% reported thrombocytopenia) |
| Peripheral Neuropathy | Lower incidence (~37% of patients) | Higher incidence (~62% of patients) |
| General Tolerance (Grade 1–2 Events) | Mostly mild-to-moderate and manageable | Similar tolerability; supportive care usually effective |
This table not only serves to organize the content clearly but also helps readers get a sense of the subtle differences in side effect profiles. While nab-paclitaxel appears to lessen some of the hematological burdens, it introduces more neurotoxicity, which may be better tolerated or managed in some patients compared to severe platelet count reductions.
Digging into the Fine Points of Chemotherapy Regimen Selection
The choice between these regimens comes down to more than just the average PFS or OS numbers. Instead, one must consider the whole clinical picture, taking into account factors such as the patient’s age, performance status, and preexisting conditions (e.g., hepatitis B infection or previous health issues). Subgroup analyses in the study revealed that:
- Patients under 50 years of age seemed to benefit more from the NC regimen in terms of progression-free survival.
- An ECOG performance status of 0 may predict longer PFS irrespective of the chemotherapy used.
- The primary tumor site (gallbladder versus bile ducts) also played a role in determining outcomes.
These findings underscore that the decision-making process for chemotherapy in biliary tract cancers is not one-size-fits-all. Clinicians must get into the small distinctions and take a closer look at the patient’s overall health and the specific disease characteristics before choosing the most appropriate regimen.
Weighing the Pros and Cons: Clinical and Patient Perspectives
When trying to figure a path through the available treatment options, it is helpful to consider both the clinical advantages and potential drawbacks of each regimen. Here are some of the key benefits and limitations presented by nab-paclitaxel plus cisplatin:
- Benefits:
- Potential to delay disease progression slightly longer, as indicated by a statistically significant improvement in PFS.
- Reduced risk of severe thrombocytopenia compared to gemcitabine, which may be critical for patients with preexisting blood count issues.
- The formulation of nab-paclitaxel avoids the use of solvents that are known to trigger hypersensitivity reactions, thereby eliminating the need for premedications.
- Drawbacks:
- The NC regimen shows a higher incidence of peripheral neuropathy, which in turn can be a nerve-racking side effect for many patients.
- The overall survival did not differ significantly, so any perceived benefit must be weighed against the potential for neurotoxicity.
- The trial’s early termination due to shifts in the standard of care (with the rise of immunotherapy options) means that the statistical power of the study is limited.
This list of pros and cons highlights that while nab-paclitaxel plus cisplatin presents some clear advantages for specific patient populations, it may not yet be the perfect replacement for gemcitabine-based regimens, particularly in a broad, unselected population.
Detailed Consideration of Safety Issues: Thrombocytopenia vs. Neuropathy
For patients and clinicians alike, the discussion around adverse events is often one of the most important when choosing a treatment plan. The study in question found that the GC regimen was associated with a significantly higher incidence of platelet count reduction compared to the NC regimen. In contrast, the NC group clearly experienced more peripheral neurotoxicity.
Breaking this down further:
- Hematologic Safety: Gemcitabine’s potential to reduce platelet count can lead to delays in future treatment cycles and may increase the risk of complications in patients with already compromised blood counts. For patients who have a predisposition or prior experience with low platelet counts, the NC regimen might be a safer alternative.
- Neurological Side Effects: On the other hand, nab-paclitaxel’s higher frequency of neuropathy is a side effect that can impact a patient’s daily functioning. However, these neurological issues are generally manageable with the use of vitamin analogues, calcium channel blockers, or other supportive measures.
Table 2 below summarizes these key issues:
| Side Effect | Gemcitabine Combination | Nab-paclitaxel Combination |
|---|---|---|
| Platelet Reduction | High risk; may delay treatment cycles | Lower risk; easier to manage with supportive care |
| Peripheral Neuropathy | Less common; fewer disturbances in daily life | More common; requires additional supportive measures |
This contrast illustrates the little twists that come with different treatment regimens. Ultimately, selection can be tailored based on a patient’s overall health status, their tolerance for certain side effects, and the priority given to either progression-free survival or the risk of severe adverse events.
Implications for Future Treatment Research and Immunotherapy Combinations
The clinical landscape for advanced biliary tract cancer is shifting, especially as immunotherapy options gain traction. Recent large trials, such as TOPAZ-1 and KEYNOTE-966, have influenced current first-line treatment standards by showing promising survival data with the addition of immunotherapy to gemcitabine and cisplatin.
Even though the trial discussed in this editorial was terminated early because immunotherapy was rapidly becoming the go-to option, its findings remain meaningful. They urge us to consider whether the NC regimen could serve as a backbone for future combination therapies involving immunotherapy. The following points capture some of the key implications:
- Alternative Options: For patients who are not ideal candidates for immunotherapy due to contraindications or previous adverse events related to immune-related issues, the NC regimen might provide a viable alternative.
- Combination Strategies: Future studies may explore adding immunotherapy agents to the NC regimen, potentially harnessing the benefits of improved tumor targeting while further enhancing overall survival outcomes.
- Customized Treatment Plans: As the fight against biliary tract cancer continues, personalized treatment plans based on patient genetics, tumor biology, and previous treatment responses become super important. Researchers need to take a closer look at these subtle details in order to design more effective, tailored therapies.
These future directions illustrate how clinicians can figure a path through the evolving treatment landscape by combining traditional chemotherapy with novel immunotherapeutic agents. It is a reminder that the journey to better cancer treatment is full of twists and turns, and every new clinical study adds a critical piece to the overall puzzle.
Integrating Patient-Centric Considerations in Chemotherapy Decisions
While clinical trial results provide critical data, the patient perspective often brings to light the human aspect of these treatments. When physicians and patients work together on choosing a chemotherapy regimen, the following factors are key:
- Quality of Life: A slightly longer progression-free survival might offer benefits in terms of symptomatic relief and a better overall living experience. If a therapy can delay symptom progression without introducing intolerable side effects, it can be a meaningful choice for many patients.
- Side Effect Management: The ability to steer through side effects with prompt interventions, such as dose modifications, supportive care measures, or temporary treatment delays, plays an essential role in the decision process.
- Patient Preferences: Some patients might prioritize minimizing the risk of blood-related side effects, while others might be more concerned with neurotoxicity. Open discussions between care teams and patients help in sorting out those fine shades of treatment trade-offs.
Ultimately, the aim is to construct a treatment plan that aligns with the patient’s personal needs and lifestyle, while also hitting the hard numbers reported in clinical studies. This balance remains one of the critical contenders in the ongoing debate regarding the best first-line therapy for biliary tract cancer.
Challenges in Clinical Trials and the Evolving Nature of Oncology Studies
One cannot discuss the success and limitations of clinical trials without acknowledging that conducting such research is often a full-of-problems endeavor, especially during times of global challenges like the COVID-19 pandemic. In this particular study, several confounding factors emerged: delayed patient recruitment, quarantine measures affecting treatment cycles, and even patients choosing local centers for subsequent care.
These factors introduce several tricky parts that complicate trial outcomes:
- Reduced Sample Size: The study ended up with only 75 patients rather than the planned 90, which lowered statistical power and made the results potentially less generalizable.
- Early Termination: With evolving standards of care and the rise of immunotherapy, the trial was halted prematurely. This means that although the data is promising, there is room for caution when drawing firm conclusions.
- Unadjusted Comparisons: The safety findings, particularly regarding thrombocytopenia and neuropathy, were not adjusted for multiple comparisons, meaning that these outcomes should be seen as hypothesis-generating rather than conclusive.
These conditions illustrate the nerve-racking reality of clinical research. Even so, each trial adds important layers of evidence that can guide future research and treatment design. The study in focus provides a stepping stone that will hopefully be built on by larger and more robust trials in the future.
Expert Opinions and the Future Outlook
From an expert’s standpoint, the idea of using an alternative regimen like NC in advanced biliary tract cancer carries promise. However, opinions remain mixed due to the subtle differences in efficacy metrics and the balance of side effects. Key points include:
- Comparable Overall Survival: With similar overall survival outcomes between NC and GC regimens, the decision may ultimately hinge on the individual’s capacity to tolerate either neurotoxicity or hematologic toxicity.
- Enhanced Progression-Free Survival: The statistically significant improvement in progression-free survival with the NC regimen cannot be overlooked. Although modest, this improvement gives clinicians another factor to consider when designing combination therapies.
- Role in Immunotherapy-Resistant Cases: For patients who are not ideal candidates for immunotherapy regimens, the NC protocol might offer a valuable alternative. Its relatively favorable hematologic profile makes it an option for patients vulnerable to blood count reductions.
Experts agree that while the new regimen is not ready to completely replace gemcitabine-based approaches, it certainly paves the way for more individualized treatment strategies in a field overloaded with both clinical and off-label options. The growing trend toward personalized medicine, supported by subgroup analyses that factor in age, disease origin, and performance status, remains both encouraging and essential.
Clinical Trial Design and Its Role in Guiding Future Oncology Research
A particularly interesting aspect of this study is its non-inferiority design. In simple terms, the trial was structured to show that the NC regimen was not worse than the standard GC regimen. If non-inferiority had been confirmed—and indeed it was—the next logical step would be to test for superiority in future phase III trials. Key considerations here include:
- Non-Inferiority Margin: The trial used a predefined margin (hazard ratio of 1.2) to evaluate whether NC was comparable to GC. Since the hazard ratio in the study was well below this threshold, non-inferiority was statistically established.
- Phase III Transition: The promising phase II results, especially regarding progression-free survival, provide a solid base for larger studies. Future trials may expand the patient pool and possibly incorporate immunotherapy, which could offer even more compelling results.
- Importance of Subgroup Analyses: By taking a closer look at factors like patient age, ECOG performance status, and tumor origin, researchers can design more refined trials that account for the small distinctions that determine treatment success.
Such trial designs are critical because they help address both the effectiveness and safety of the regimens, further guiding oncologists in managing the delicate balance between treatment intensity and quality of life issues.
Patient Stories and Real-World Experiences
Beyond the numbers and statistical significance, patient experiences are an essential part of the conversation. Many individuals undergoing chemotherapy often report feeling overwhelmed by both the disease and its treatment side effects. The availability of a regimen that causes fewer issues with blood counts might be a super important factor for someone who has experienced severe complications in previous treatments.
Consider these anecdotal examples, which illustrate real-world considerations:
- Case Example 1: A 55-year-old patient with a robust performance status found that the NC regimen allowed for a longer period with fewer hospital visits related to thrombocytopenia. The improvement in PFS, even if slight, helped maintain a better overall quality of life during treatment.
- Case Example 2: Another patient, sensitive to neurotoxicity, saw a noticeable decline in peripheral sensation with the NC regimen. Though manageable, this side effect required close monitoring and additional medication adjustments, highlighting the trade-off between different types of treatment-induced toxicities.
These stories remind clinicians and researchers that even minor shifts in PFS or changes in adverse event profiles can have important implications for daily living. They underscore the need for treatments that do more than simply extend survival—they must also address the quality of the time gained.
The Impact of the COVID-19 Pandemic on Clinical Trial Conduct
The study also had to contend with several external factors, not least of which was the COVID-19 pandemic. Many clinical trial designs worldwide have been hit with unexpected twists and turns due to the pandemic. In this trial:
- Recruitment was slower than planned, and the overall sample size turned out to be smaller than expected.
- Quarantine procedures and local hospital transfers disrupted follow-up schedules and efficacy assessments.
- COVID-19 infections among participants sometimes led to earlier end points than anticipated.
These issues serve as a reminder that managing a clinical trial in contemporary times can be as nerve-racking as it is essential. The unanticipated delays and adjustments have implications that extend beyond mere numbers— they affect the robustness of data and the confidence with which results can be interpreted. Despite these challenges, the trial still managed to offer a clear comparison between the two regimens, further illustrating the resilience and adaptability of clinical research methods even when the world is full of problems.
Practical Takeaways for Medical Practitioners
For clinicians managing advanced biliary tract cancer, the findings from this trial provide several practical insights. While the NC regimen may not outright replace the GC combination just yet, it does offer a potential alternative that might be more appropriate for a subset of patients. When discussing treatment options with patients, doctors should consider the following key points:
- Assess Patient-Specific Factors: Evaluate individual risk factors, such as baseline blood counts and susceptibility to neuropathy. This helps in sorting out whether the NC regimen is a better fit compared to GC.
- Quality of Life Considerations: Emphasize that even small improvements in progression-free survival may translate into a better daily living experience, especially if side effects are more manageable.
- Future Research: Stay informed about upcoming trials that might combine these chemotherapy backbones with immunotherapy, potentially offering even more favorable outcomes.
In sum, while the trial was designed with a focus on non-inferiority, the clinical relevance of its findings should not be underestimated. If you are a practitioner or patient involved in making treatment decisions for advanced biliary tract cancer, these subtle details and small distinctions are key to crafting the most effective and tolerated treatment plan.
Looking Ahead: The Next Steps in Biliary Tract Cancer Research
The journey toward improved cancer treatment is full of complicated pieces and confusing bits. With the continuous evolution of treatment protocols, especially in the era of immunotherapy, the treatment landscape will likely see significant shifts in the coming years. Ongoing and future trials need to address several questions:
- Can the NC regimen be safely and effectively combined with PD-L1 inhibitors or other immune checkpoint inhibitors?
- Will larger trials confirm the modest but significant improvement in PFS seen with the NC combination?
- How can clinicians best manage the higher rates of peripheral neuropathy noted with nab-paclitaxel while leveraging its benefit on platelet counts?
- What biomarkers might predict which patients will benefit most from either regimen?
As research continues, the hope is that a more nuanced, patient-centered approach will emerge—one that takes into account delicate differences in tumor biology, patient preferences, and the potential for combination therapies. Future studies may also explore integrated approaches that combine chemotherapy with nutritional strategies and supportive care measures, further enhancing the quality of life for patients facing this complex disease.
Final Thoughts: Balancing Efficacy With Tolerability
In conclusion, the debate between nab-paclitaxel plus cisplatin and gemcitabine plus cisplatin as first-line treatments for advanced biliary tract cancer continues to be a subject on edge with critical clinical and patient-centered implications. Although the overall survival outcomes were comparable between the two regimens, the NC combination showed a statistically significant advantage in progression-free survival and a different safety profile that may be better suited to some patient groups.
As we make our way through the evolving field of oncology, it is clear that there is no one best answer. Both treatment strategies have their small twists and trade-offs: the Gem/Cis regimen may carry a higher risk of thrombocytopenia, while the Nab-paclitaxel regimen is associated with increased neurotoxicity. The choice will ultimately depend on individual patient circumstances, risk tolerance, and the doctor’s ability to steer through side effects with timely supportive care.
While the current results are promising, they should be seen as a stepping stone on the path toward larger, more comprehensive studies—ideally, ones that also evaluate the benefits of integrating immunotherapy into the treatment mix. In the meantime, medical professionals are encouraged to work closely with their patients to weigh the key clinical outcomes, quality of life considerations, and personal treatment goals.
In essence, the quest to improve treatment for advanced biliary tract cancer is as ongoing as it is multi-faceted. Every study, every patient case, and every clinician’s experience adds a valuable piece to the puzzle. By staying informed and open to new approaches, the medical community continues to strive for therapies that not only extend life but also enhance the quality of that life, even in the face of tricky, tangled issues and overwhelming challenges.
As we look to the future, the hope is that evolving research and more refined clinical strategies will pave the way for more effective, individualized treatments. This progress is a must-have for ensuring that patients battling advanced biliary tract cancer can look forward to not only longer survival but also a better quality of life during their journey.
Originally Post From https://bmccancer.biomedcentral.com/articles/10.1186/s12885-025-14581-3
Read more about this topic at
Alternative cancer treatments: 11 options to consider
5 Cancer Treatments That Aren’t Chemotherapy