Giredestrant and a New Dawn for ER+ Breast Cancer Treatment: An Editorial Perspective
The recent phase 3 evERA study has sparked a lively discussion in the oncology community. As an editor with a passion for modern medicine and patient-centered care, I find it refreshing to see a novel oral regimen making headway in a field that has long struggled with tricky parts in treating ER-positive, HER2-negative breast cancer. In this opinion editorial, I will dig into the study’s design, outcomes, and broader implications for patients who have exhausted other treatment pathways.
Over the past few years, advancements in endocrine therapy have moved the needle in managing breast cancer, yet not without facing a series of tangled issues. The evERA study, which evaluated giredestrant plus everolimus compared to the physician’s choice standard-of-care therapies, represents an important step forward. Its positive results in enhancing progression-free survival (PFS) have given hope to many patients, clinicians, and researchers alike.
Emerging Evidence in the Fight Against Advanced ER-Positive Breast Cancer
Recent years have seen a significant shift as the oncology arena works through the confusing bits of endocrine resistance. Traditional treatments, including various endocrine therapies and CDK4/6 inhibitors, have improved outcomes, but there remains a pressing need for more effective treatment options for patients whose cancer has become resistant. The evERA study’s results, pointing to a statistically significant improvement in PFS for both the entire intent-to-treat population and specifically for those with ESR1 mutations, are a breath of fresh air.
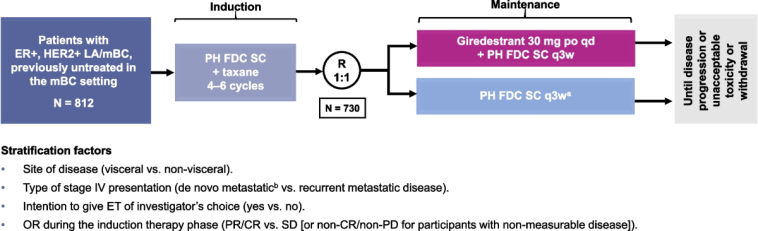
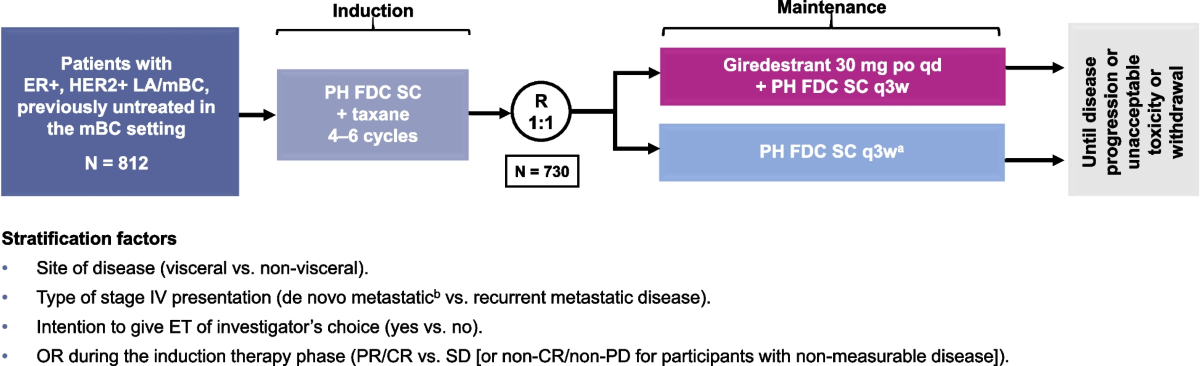
This study enrolled 373 patients with locally advanced, unresectable, or metastatic disease. By comparing an all-oral selective estrogen receptor degrader (SERD) regimen to conventional endocrine therapies such as exemestane, fulvestrant, or tamoxifen – each combined with everolimus – the trial has managed to get into the fine points of how targeted therapies can be more effective when paired correctly. In a landscape where every incremental benefit counts, these findings are super important because they offer hope to a large percentage of patients whose options were running low.
Understanding the Mechanism: How Does Giredestrant Work?
At its core, giredestrant is designed to degrade the estrogen receptor that fuels the growth of many breast cancer cells. Unlike traditional SERDs, which are usually injectable and sometimes face issues with patient compliance, giredestrant is all-oral, offering a more convenient and potentially more effective approach. This advantage is not merely practical but also critical, as it may help patients manage their treatment routine more effectively.
When combined with everolimus—an inhibitor of the mTOR pathway known for its role in cell proliferation—the regimen works through multiple pathways to slow down tumor progression. Everolimus has been used successfully in several cancer settings, and its inclusion in the combination therapy helps to address some of the little details that cause resistance to treatment. The dual-mode action, therefore, is poised to overcome some of the nerve-racking complexities that have long plagued endocrine-resistant breast cancers.
Digging Into the Progression-Free Survival Findings
The study’s primary endpoint was progression-free survival, a critical metric that reflects the time during and after treatment that a patient lives with the disease without it getting worse. The improvement in PFS seen in the evERA study is not only statistically significant but also clinically meaningful. This means that giredestrant, in combination with everolimus, offers patients an extra layer of defense against the rapid progression of their disease.
For many patients, facing a long line of treatments after failing to respond to a CDK inhibitor can be overwhelming. The concept of extended progression-free survival brings a sense of hope as it can directly impact overall survival expectations, even though overall survival data were still immature at the time of analysis. In many ways, these findings help untangle some of the confusing bits associated with endocrine resistance, and they may well refashion the standard of care for advanced ER-positive breast cancer.
Analyzing Tolerability and Safety: What Does It Mean for Patients?
While efficacy is a major concern, safety and tolerability remain paramount in any treatment regimen, especially when dealing with patients who have already been through multiple lines of therapy. One of the reassuring aspects of the evERA study is that the giredestrant combination was well tolerated. The observed adverse events were consistent with what is already known from the individual safety profiles of both giredestrant and everolimus.
This is particularly important because achieving a balance between effective disease control and manageable side effects is critical in oncology. For many patients, the decision to continue aggressive treatment is influenced as much by the side effects as by the potential benefits. Here, the new regimen seems to offer a favorable profile, minimizing additional complications that could stem from already overwhelming treatment histories. In short, the therapy helps patients find their path to treatment without adding too many nerve-racking new challenges.
Key Data Points and Trial Design Elements
The trial was conducted across 171 global sites with well-defined eligibility criteria:
- Enrollment of patients with locally advanced unresectable or metastatic ER-positive, HER2-negative tumors.
- Prior treatment with endocrine therapy in combination with a CDK4/6 inhibitor in either metastatic or adjuvant settings.
- Exclusion of patients with significant cardiac issues or uncontrolled central nervous system metastases, ensuring safety.
The study’s design factors aimed to steer through many of the tricky parts related to patient heterogeneity. By randomizing patients to receive either the giredestrant regimen or one of the standard therapies, researchers are poised to glean a clear comparison. Secondary endpoints such as overall survival (OS), objective response rate, duration of response, and quality of life metrics further deepen our understanding of the treatment’s potential.
Examining the Broader Implications for Clinical Practice
While the immediate clinical relevance of the trial data is significant, broader implications related to how we treat advanced ER-positive breast cancer are equally super important. For a condition that represents nearly 70% of breast cancer cases, finding new therapeutic avenues is not just beneficial—it’s critical. Oncology professionals are now tasked with integrating these findings into everyday practice, which involves understanding the subtle details and fine points of how this novel treatment performs in real-world settings.
From a clinician’s perspective, these findings could pave the way toward revising treatment guidelines and expanding the therapeutic horizon for patients who previously had limited options. As more data are shared at upcoming medical meetings and regulatory discussions, the ripple effect could extend into community oncology practices, ultimately improving patient outcomes across the board.
Our Take on the Regulatory and Market Impacts
The evERA study has already stirred conversation among regulators and pharmaceutical companies alike. Given that overall survival data are still maturing, regulatory authorities will need to carefully assess whether the improvements in progression-free survival justify full approval in the short term. However, there is a positive trend given the robust design and promising interim data, and several stakeholders are optimistic that these results will eventually translate to a new standard for patients.
There is also an economic dimension to this discussion. An all-oral regimen is not only convenient for patients but may also be attractive to payers who favor outpatient care over costly inpatient interventions. As the healthcare system becomes increasingly sensitive to both clinical benefits and cost-effectiveness, treatments like this could shape market dynamics in significant ways. To summarize some critical perspectives:
| Perspective | Implications |
|---|---|
| Clinical | Improved PFS and potential overall survival benefits offer new hope for patients. |
| Economic | Convenience of an all-oral regimen could reduce hospitalization costs and increase treatment adherence. |
| Regulatory | Promising interim results may drive faster review processes and modifications in treatment guidelines. |
Patient-Centered Perspectives: Balancing Efficacy and Quality of Life
From the viewpoint of those living with advanced breast cancer, the impact of any new treatment goes far beyond traditional clinical endpoints. Extended progression-free survival offers not just more time but also an enhanced quality of life—a possibility that resonates strongly with patients who have already navigated many intimidating twists and turns in their treatment journey.
The giredestrant combination’s favorable safety profile suggests that patients need not worry excessively about new, off-putting side effects. Instead, they can focus on the possibility of a treatment that is both effective and tolerable. For many, the idea of switching to an oral therapy, which can be taken at home and integrated into daily life more easily than intravenous regimens, represents a significant step forward. This is a key aspect when considering overall treatment success: therapy that patients can manage without facing additional hurdles that complicate day-to-day living.
Decoding the Fine Points of Endocrine Therapy Resistance
One of the most challenging aspects of managing ER-positive breast cancer is overcoming resistance to endocrine therapy. Clinicians are all too familiar with the difficult parts of treating a disease that evolves and adapts, often rendering standard treatments less effective over time. The evERA study’s focus on patients previously treated with endocrine therapy in combination with a CDK4/6 inhibitor highlights an area that has been riddled with obstacles.
These obstacles include the subtle differences in tumor biology that can lead to resistance, as well as the tangled issues associated with long-term therapy. By providing an alternative approach through giredestrant—a drug specifically designed to degrade the estrogen receptor—the study offers a new tool to counteract these challenges. The ability to tackle endocrine resistance head-on, with a regimen that patients can manage more easily, is a notable advancement in cancer care.
Optimizing Treatment Strategies: Clinical Trial Design and Patient Selection
Every clinical trial faces various twists and turns related to study design and patient selection. The evERA study appears to have carefully worked through these challenging aspects by employing strict eligibility criteria, robust randomization, and comprehensive secondary endpoints. Such a design ensures that the overall results are both meaningful and applicable to real-world settings.
Some of the organized strategies used by the study include:
- Global Recruitment: The use of over 170 sites worldwide ensures a diverse patient population that reflects the broader demographics of ER-positive breast cancer.
- Stringent Eligibility Criteria: Ensuring patients have not received more than two prior lines of systemic endocrine therapy and excluding those with significant cardiac issues helps isolate the effects of the treatment regimen.
- Multiple Endpoints: By evaluating not only progression-free survival but also response rates, duration of response, and quality of life, the study provides a multifaceted look at the benefits of the new therapy.
This thoughtful design process is key to getting around the nerve-racking challenges associated with clinical trials, and it helps clinicians gain confidence in applying the findings to everyday practice.
Future Directions in Endocrine Therapy and Personalized Treatment Approaches
While the enthusiasm regarding giredestrant is understandable, it is essential to consider the bigger picture. The evolution of endocrine therapy for breast cancer is a continuously moving target. With the advancements we are seeing today, future treatment strategies may well incorporate personalized approaches that combine multiple modalities—targeted therapies, immunotherapy, and perhaps even genetic profiling—to optimize patient outcomes.
In the near future, one may envision a treatment model where the specific characteristics of a patient’s tumor, including details like ESR1 mutations, guide the selection of combined therapies. The giredestrant study is an early step in that direction, showing that an all-oral regimen can be effective even in cases where traditional endocrine therapies have failed. Potential future research directions include:
- Comparative Studies: Direct comparisons between giredestrant-based regimens and other emerging oral SERDs to determine the optimal choice for individual patients.
- Biomarker Exploration: Deep dives into the fine shades of molecular signals that predict response to therapy, thus allowing for more tailored treatment strategies.
- Combination Trials: Evaluations of giredestrant in combination with other targeted agents or even immunotherapies, aiming to address the full gamut of challenges posed by endocrine resistance.
Each of these avenues of research seeks to figure a path toward even more effective treatment paradigms. With increasing personalization, the once broad-brush approach to cancer therapy may give way to a more nuanced, individualized treatment plan, maximizing both survival and quality of life.
Addressing the Practical Concerns: Cost-Effectiveness and Patient Compliance
As we work through the evolving landscape of treatment options, it is important to consider the practical aspects of new therapies. While clinical efficacy is paramount, the economic burden of treatment and patient compliance are equally critical, especially in resource-limited settings. The all-oral nature of the giredestrant regimen makes it easier for patients to incorporate the therapy into their daily routine without the need for frequent hospital visits.
This ease of administration can translate into:
- Lower Healthcare Costs: Reduced need for intravenous administration can lessen the financial load on both the healthcare system and the patient.
- Improved Patient Adherence: Oral therapies can often be taken on schedule more reliably, which can contribute to better overall outcomes.
- Enhanced Quality of Life: The convenience of home-based oral therapy helps patients maintain a better sense of normalcy during treatment.
These factors are critical when discussing the overall impact of new treatment options, as they address not just the clinical outcomes but also the day-to-day experiences of patients.
Insights from the Oncology Community: Clinical and Research Perspectives
The oncology community has largely welcomed the evERA study’s findings, with researchers and clinicians alike praising the innovative approach to overcoming the tricky parts of endocrine resistance. Experts in medical circles are now busy sorting out the details of how best to integrate these new findings into established treatment protocols. In discussions at recent conferences and peer-to-peer rounds, several key themes have emerged:
- Hope Amidst Challenges: The study demonstrates meaningful benefits even in a patient population that has limited options after standard therapies fail.
- Balanced Risk-Benefit Considerations: The favorable safety profile paired with improved efficacy makes this approach a promising candidate for broader adoption.
- Call for Further Research: While the initial data are promising, additional studies focusing on long-term outcomes and overall survival are eagerly awaited.
These discussions reflect a broader shift toward treatments that are not only effective but also flexible and easier for patients to manage on a day-to-day basis. Clinical testimonies and emerging data from real-world settings will further help shape how these therapies are used in the future.
Integrating New Therapies into Existing Treatment Pathways
The big question facing oncologists now is how to effectively integrate innovations like giredestrant into the current treatment landscape. With many patients already exposed to multiple lines of therapy, this new regimen must fit into a broader framework without adding extra layers of complexity. Fortunately, its all-oral format eases this integration.
Key considerations include:
- Treatment Sequencing: Determining the optimal sequence of therapies to maximize patient benefit and delay resistance.
- Combination Approaches: Evaluating how giredestrant works alongside other agents, such as mTOR inhibitors like everolimus, for synergistic effects.
- Patient-Specific Factors: Incorporating biomarkers such as ESR1 mutations into the decision-making process to ensure that each treatment is as individualized as possible.
By addressing these practical issues, the oncology community can better figure a path toward seamless integration of novel therapies with traditional treatment pathways. The ultimate goal remains—improving patient outcomes and quality of care.
Weighing the Risks and Rewards: Clinical Decision-Making in a Complex Landscape
Clinical decision-making in oncology is often a balancing act, requiring practitioners to weigh the benefits of a new therapy against its potential risks. The evERA study offers a promising new option, but as with all therapies, there are still unanswered questions. How will the improved progression-free survival translate into overall survival benefits? Will the combination therapy hold up over time in a broader patient population? And importantly, how will it compare to future therapies currently under development?
While these questions are not trivial, the initial evidence suggests that giredestrant in combination with everolimus could soon become one of the key components in the toolkit of modern oncologists. In a landscape replete with challenging twists and turns, an option that is both effective and manageable for patients carries significant weight.
Future Clinical Trials and Research Opportunities
The completion of the evERA study, with a planned end date of October 15, 2026, represents only one juncture in a long journey toward refining breast cancer treatment. Future clinical trials will likely explore:
- Head-to-Head Comparisons: Directly comparing giredestrant-based regimens with other novel therapies will help establish clear guidelines for clinical practice.
- Combination Strategies: Further investigations into combining oral SERDs with other targeted agents, such as CDK4/6 inhibitors or immunotherapies, to reveal potential synergistic benefits.
- Biomarker-Driven Approaches: Advances in genomic testing might allow for more precise patient selection, ensuring that each individual receives the treatment most likely to be effective based on the fine shades of their tumor biology.
These future research avenues are essential to fully unpack the potential of giredestrant and to further improve outcomes for a patient population that has long needed new, effective options. With continued exploration and discussion among clinical experts, the landscape of advanced ER-positive breast cancer treatment is set to evolve rapidly.
Conclusion: A Step Forward in a Challenging Journey
In conclusion, the evERA study’s promising results with the giredestrant and everolimus combination mark a notable evolution in the treatment of advanced ER-positive, HER2-negative breast cancer. Faced with a condition that has been burdened by tricky parts and overwhelming odds after standard therapies, patients now have renewed hope that more effective and manageable treatment options are on the horizon.
As we continue to work through the complexities of endocrine resistance, this novel treatment underscores the importance of innovative approaches that consider both clinical efficacy and quality of life. While further research will be necessary to fully establish long-term benefits, it is clear that efforts like these are a pivotal step toward steering through the increasingly complicated pieces of breast cancer management.
For clinicians, researchers, and patients alike, the journey has only just begun. With continued advances and a commitment to exploring every subtle detail of treatment response, the future of breast cancer care promises to be brighter—and, most importantly, more personalized. In a field filled with nerve-racking challenges and loads of practical issues, success lies in our ability to find our way through with an open mind and a willingness to adapt.
Ultimately, giredestrant’s promising clinical data invites us to reimagine what is possible in the realm of endocrine therapy. It reminds us that, despite the many twists and turns, the pursuit of improved outcomes for patients remains the super important mission at the heart of modern oncology. Should these encouraging results continue to translate into long-term benefits, we may soon see a transformation in treatment guidelines and patient care, changing the story of advanced ER-positive breast cancer for the better.
Key Takeaways
To summarize some of the most essential points from our discussion:
- Innovative Approach: Giredestrant’s all-oral regimen combined with everolimus introduces a new way to address endocrine resistance.
- Improved PFS: Statistically significant improvements in progression-free survival offer tangible benefits in disease management.
- Safety Profile: The manageable side effects of this combination therapy contribute to its viability as a long-term treatment option.
- Patient Convenience: Oral administration provides an ease of use that can ultimately improve adherence and quality of life.
- Future Research: Ongoing and future trials will be critical in unpacking the full clinical impact, especially regarding overall survival and personalization of treatment.
It is in this spirit of cautious optimism and evidence-based advancement that we now look toward a future where each patient’s journey can be met with treatments tailored to their individual challenges and needs.
Final Thoughts
In our quest to provide better care for patients with advanced breast cancer, research efforts like the evERA study remind us that progress is a collaborative journey. Every study, every trial, and every patient experience contributes to a collective effort toward making the once nerve-racking world of cancer treatment a little less intimidating and a lot more promising.
While challenges remain, the data we now have reinforce the notion that innovative, patient-friendly therapies are not a distant dream but a reality in progress. As we continue to get into the research and keep a close eye on emerging data, one thing remains clear: progress in oncology is full of hope, and every step forward is a win for patients, healthcare providers, and the scientific community at large.
For those who have followed the evolution of endocrine therapies over the years, today’s findings represent a significant milestone. It is a call to arms for more research, deeper collaboration, and above all, a steadfast commitment to improving patient outcomes—one innovative treatment at a time.
Originally Post From https://www.targetedonc.com/view/giredestrant-improves-survival-in-er-her2-breast-cancer
Read more about this topic at
Molecular Basis of HER2-Targeted Therapy for …
TRIUMPH Study: A multicenter Phase II study to evaluate …