Giredestrant’s Breakthrough in Early-Stage Breast Cancer: An Opinion Editorial
The oncology field is witnessing an exciting shift as emerging evidence suggests that giredestrant, a novel oral selective estrogen receptor degrader (SERD), might herald a new era for early-stage breast cancer treatment. Recent phase 3 trial results from the lidERA study have shown an improvement in invasive disease-free survival (DFS) among patients with estrogen receptor (ER)–positive, HER2-negative early-stage breast cancer compared to standard-of-care therapies. In this editorial, we take a closer look at these developments, weigh the benefits against potential risks, and explore how this breakthrough could reshape the future of endocrine treatments in oncology.
Before we begin our discussion, it is important to understand that ongoing clinical advances are continuously reshaping our approach to cancer treatment. With breast cancer representing nearly 70% of all diagnoses in ER-positive cases, any innovation in this area is both essential and timely. The results of the giredestrant trial provide a window into what might soon become an indispensable tool in oncologists’ arsenals. Let’s dive into the details and address the tricky parts, tangled issues, and potential twists and turns that come with integrating a new drug into clinical practice.
Longer-Term Invasive DFS Improvement and Its Impact on Patient Outcomes
One of the most compelling outcomes of the lidERA trial is the statistically significant and clinically meaningful improvement in invasive DFS. In simpler terms, patients receiving giredestrant experienced a longer period without invasive recurrence when compared to those undergoing standard adjuvant endocrine therapy. This is notable because invasive DFS serves as an early indicator of treatment benefit before overall survival (OS) data mature into a clear picture. Although the overall survival data are still immature, the trial has already recorded encouraging positive trends.
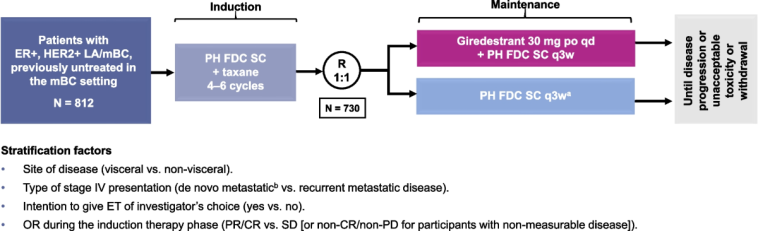
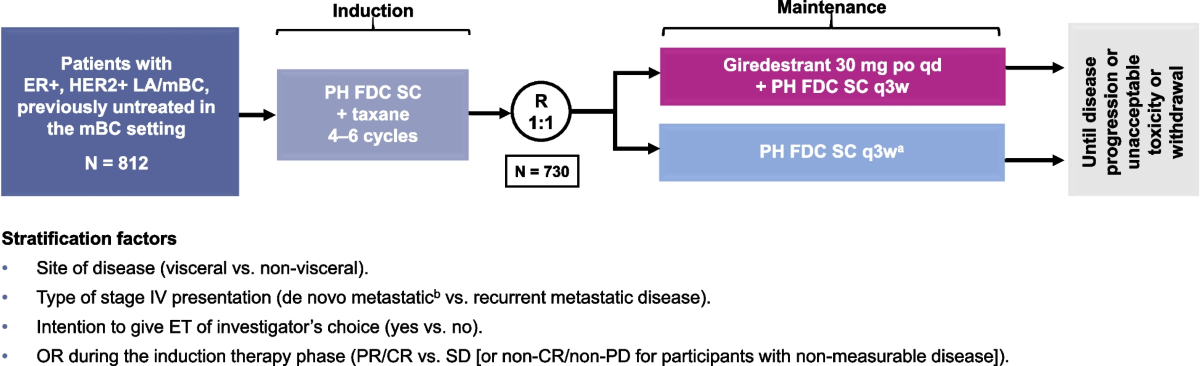
Improvement in DFS is especially critical in the adjuvant setting. For patients with early-stage breast cancer, achieving a longer DFS provides more time before any potential recurrence occurs, allowing space for recovery and enhancing quality of life. The trial design encompassed more than 4,100 patients with intermediate- to high-risk stage I to III ER-positive, HER2-negative breast cancer, making the data robust and representative of the patient population. A key takeaway is that giredestrant holds the promise of becoming a new key endocrine therapy that not only extends DFS but might also improve long-term outcomes in a large subset of breast cancer patients.
Understanding Patient Selection and Giredestrant’s Safety Profile
The safety profile of any new therapy is a major factor in its clinical adoption. Here, giredestrant appears to have a well-tolerated safety profile, with adverse effects (AEs) that align with those previously seen in its known profile. No unexpected or severe toxicities were reported, which suggests that the regimen does not bring with it any additional nerve-racking hurdles for patients. This is particularly encouraging in light of the often intimidating side effects associated with some endocrine therapies.
Prior to enrolling in the lidERA study, patients underwent careful selection to ensure that only those who could benefit most from the therapy were included. Criteria such as documented ER-positive, HER2-negative disease, completion of definitive surgery, and a minimum washout period post-chemotherapy were critical. Additionally, individuals with active cardiac disease, significant renal dysfunction, or prior malignancies were excluded. These measures illustrate not only the importance of patient safety but also the efforts made to target a group that is most likely to exhibit treatment benefit.
The trial’s design also aimed to minimize confusing bits and tangled issues related to cross-therapy interference. Through clear inclusion and exclusion parameters, the investigators sought to create a patient cohort that would yield reliable results while safeguarding against potential complications. This methodical approach is a prime example of how modern clinical trials strive to steer through conflicting variables and focus on the nitty-gritty details that matter to both clinicians and patients.
Comparing Oral SERDs to Standard Endocrine Therapies: A Closer Look
Current standard-of-care (SOC) endocrine therapies, largely comprised of third-generation aromatase inhibitors like letrozole, anastrozole, and exemestane, have been the mainstay of adjuvant treatment for patients with ER-positive, HER2-negative breast cancer. However, resistance to these therapies remains a significant challenge, leading many clinicians to seek alternatives that can better manage disease progression.
Oral SERDs, such as giredestrant, work by binding to estrogen receptors and facilitating their degradation. This mechanism not only blocks estrogen signaling but also downregulates the number of receptors available for activation. The lidERA study’s design, which compared a daily dosage of 30 mg giredestrant to SOC therapy, offers key insights into how these novel drugs stack up against conventional treatments. In clinical practice, achieving greater efficacy while maintaining a manageable side effect profile is of utmost importance.
For clinicians and patients alike, the decision to switch to or integrate a new therapy is tempered by concerns over managing the tricky parts associated with novel intersection points. Some of these challenges include:
- Understanding the subtle parts of molecular signaling pathways.
- Balancing the new drug’s efficacy with any potential overlapping toxicities.
- Figuring a path through the myriad of existing treatment options and patient preferences.
This comparison between oral SERDs and standard endocrine therapies is a topic that continues to attract attention. The expanded data from previous phase 2 studies, such as the coopERA trial, have shown that giredestrant can reduce biomarkers like Ki67 to a greater extent than aromatase inhibitors. Such findings underscore the potential for giredestrant to become a super important tool in the oncologist’s armamentarium.
Overcoming the Tricky Parts of Clinical Trial Design
Clinical trials, by their very nature, are loaded with challenges that range from patient selection and trial design fluctuations to managing unexpected adverse effects. The lidERA trial managed to steer through these potential pitfalls by focusing on clear primary and secondary endpoints. The primary endpoint was invasive DFS, supported by multiple secondary endpoints including overall survival, locoregional recurrence, distant recurrence-free interval, quality of life, and safety.
Trial designers had to get into a range of topics to ensure the study was both rigorous and clinically applicable. Some of the tricky parts they encountered and managed include:
- Tangled issues with washout periods: Ensuring patients had sufficient time after completion of chemotherapy before starting adjuvant therapy was essential to avoid confounding effects.
- Complicated pieces of patient eligibility: By excluding those with recent malignancies or those treated with CDK4/6 inhibitors, the researchers aimed to deliver clean and interpretable results.
- Overcoming the fine points of safety monitoring: With continuous surveillance of adverse events, the trial successfully demonstrated that giredestrant’s safety is in line with known endocrine therapy profiles.
The careful and systematic approach used in the lidERA trial not only provided robust data on giredestrant but also offered a blueprint for future studies. In a field where every decision is loaded with issues and potential setbacks, such methodical planning is key.
Regulatory Hurdles and The Path to Approval
Every new treatment must first pass through the rigorous scrutiny of regulatory bodies before it can become a part of everyday clinical practice. The encouraging results from the lidERA trial have indeed positioned giredestrant as a strong candidate for regulatory review. Given its potential to improve invasive DFS and extend the disease-free interval, health authorities might consider this therapy for expedited or fast-track designations.
Before any regulatory approval, it is essential that additional data—especially mature overall survival and long-term safety profiles—are closely monitored and reviewed. The regulatory process is often a nerve-racking experience for pharmaceutical companies and clinicians alike, as even promising therapies must demonstrate improvements against stringent benchmarks. Nevertheless, should giredestrant gain regulatory approval, it might not only change current treatment guidelines but also encourage further innovation in other areas of oncology.
Regulatory approval could lead to a domino effect on clinical practice. With a new option available, clinicians could start to adjust treatment protocols and possibly even reclassify what is considered standard care in the adjuvant setting. This could reassure many patients who face the daunting prospect of returning cancer and allow them to manage their treatment with a therapy that has already demonstrated significant benefits in early-stage disease.
Learning from Past Trials and Applying New Knowledge
History has taught us that each new breakthrough in cancer therapy builds upon past successes—as well as lessons learned from previous setbacks. The promising data from the phase 2 coopERA trial, which highlighted giredestrant’s ability to offer a better reduction in Ki67 levels compared to aromatase inhibitors, laid the foundation for the larger lidERA trial. Meanwhile, results from the phase 3 evERA trial in advanced breast cancer further solidify the potential of giredestrant in various settings of the disease continuum.
In these trials, it is important to note the fine shades of differences in patient responses. Although every study is designed to minimize hidden complexities, subtle details—such as the degree of receptor degradation or slight differences in side effect profiles—can influence overall efficacy and patient satisfaction. Clinicians must, therefore, get into these aspects to better understand which patient groups stand to benefit most. We find that such thorough cross-trial analyses serve as an essential resource for tailoring treatment strategies.
Opportunities for Integration in Multidisciplinary Care
The integration of new drugs like giredestrant into current treatment regimens is a multifaceted process. Beyond merely replacing a standard therapy, it involves a broader rethinking of clinical workflows and multidisciplinary collaboration. Surgeons, medical oncologists, pathologists, and radiologists all contribute to the decision-making process, especially when it comes to implementing novel therapies in real-world settings.
For instance, an oncology team might consider the following critical steps for successful integration:
- Coordinated patient management: Frequent discussions among team members help address any confusing bits regarding treatment sequencing and side effect management.
- Monitoring and follow-up: Establishing a routine for monitoring patient responses can enhance early identification of any adverse effects or resistance patterns.
- Education and training: Continuous learning initiatives through conferences, workshops, and online modules ensure that every member of the care team is up-to-date with the latest research findings.
By piecing together input from various specialties, the oncology community can work together to figure a path through the complex landscape of cancer treatment, ensuring that breakthroughs such as giredestrant benefit patients in the most efficient and thoughtful way possible.
Future Directions and Evolving Treatment Paradigms
Looking ahead, the future of endocrine therapy in breast cancer appears increasingly optimistic. The data supporting giredestrant’s efficacy in early-stage breast cancer has already spurred discussions about its potential role in other breast cancer subtypes and even in combination with other targeted agents. As more research is conducted, areas that merit closer examination include:
- Combinatorial strategies: Exploring how giredestrant might pair with other drugs—like CDK4/6 inhibitors or immunotherapeutic agents—to address both early and advanced breast cancer.
- Molecular profiling: Using biomarkers and detailed patient profiling to identify those who are more likely to benefit from oral SERD therapy.
- Quality of life considerations: Research into patient-reported outcomes will be critical in assessing the full value of giredestrant beyond traditional endpoints.
As clinicians continue to figure a path through the evolving treatment landscape, the role of multidisciplinary board discussions and precision oncology meetings will be crucial. These sessions not only provide insights into the small distinctions in patient presentations but also help steer through the often intimidating maze of treatment options available today.
In the context of rapidly shifting medical paradigms, the introduction of oral SERDs could be seen as a pivotal moment. The ability to offer a well-tolerated, orally administered therapy that improves DFS in early-stage disease is a super important development, potentially changing treatment standards across the board.
Discussing the Tricky Parts of Clinical Adoption and Real-World Implementation
Implementing a new therapy like giredestrant into the routine care of cancer patients is not without its challenges. Translating clinical trial findings into everyday practice involves figuring out a path through several tricky parts and managing the occasional unexpected twist. Some of the key challenges include:
- Education of healthcare providers: Doctors, nurses, and allied health professionals need to be brought up to speed on the mechanisms, benefits, and potential side effects of giredestrant. This upskilling effort is essential to ensure widespread, safe, and effective use.
- Patient communication: When introducing any new treatment, it is critical to clearly explain the benefits and potential risks. Patients must be given a comprehensive understanding of how giredestrant differs from existing therapies, thus making their decision-making process less overwhelming.
- Cost and reimbursement: New therapies often come with higher costs. Addressing issues related to insurance coverage and potential financial burdens for patients is a key part of the real-world implementation process.
- Management of side effects: Establishing clear protocols for the management of adverse effects is critical. Although giredestrant appears to be well-tolerated, every new treatment introduces its own set of challenges that must be carefully managed in clinical settings.
Effective overcoming of these challenges will require efforts from multiple stakeholders, including pharmaceutical companies, healthcare providers, patient advocacy groups, and policy makers. Collaboration and open communication channels can help ensure that the advantages demonstrated in clinical trials translate smoothly into clinical practice.
Expert Perspectives and Multidisciplinary Insights
It is worth noting that influential voices within the oncology community, including chief medical officers and global product development leads, have expressed optimism over giredestrant’s potential. For example, leaders at Roche have highlighted not only the benefit in invasive DFS but also the broader impact such a breakthrough could have on the treatment landscape for ER-positive breast cancer.
Experts emphasize that while the current findings are promising, continuous research and in-depth discussion are necessary to address the subtle details that separate incremental improvements from groundbreaking changes. Here are some quick bullet points summarizing expert opinions:
- Many believe that the clear improvement in DFS represents a turning point in early-stage hormone receptor–positive breast cancer management.
- The turnover of traditional aromatase inhibitors with more modern oral SERDs may set a precedent in oncology treatment protocols.
- There is a strong call for further studies to better understand the long-term benefits and potential combination strategies with other anticancer agents.
- Continuous monitoring and post-marketing data collection will be necessary to firmly establish the risk–benefit profile of giredestrant.
These insights serve as a reminder that while promising, the integration of giredestrant into standard care must be approached with a balanced perspective that appreciates both its potential and the challenges inherent to any new therapeutic strategy.
Implications for Patients and Their Families
The promise of a new oral SERD that can enhance invasive DFS is more than just a statistical milestone—it represents hope for thousands of patients and their loved ones. For patients facing the daunting prospect of aggressive breast cancer treatment regimes, a shift toward more tolerable yet effective therapies can significantly improve quality of life.
Many patients find the technical language of clinical studies off-putting, yet the core message is one of progress and increased possibility. Here are some ways in which giredestrant might benefit real-world patients:
- Longer periods without invasive disease: This offers patients more time to recover and potentially focus on life after cancer treatment.
- Improved treatment tolerance: A manageable side effect profile helps reduce the nerve-racking fear associated with adverse reactions.
- Enhanced patient confidence: Knowing that there are innovative treatment options available can foster hope and diminish the overwhelming burden of a cancer diagnosis.
For families, these advances provide reassurance that the medical community is continuously working to bring less intimidating solutions to serious health problems. A treatment that promises both efficacy and quality of life improvements has the potential to change the discourse around what is possible in cancer recovery.
The Road Ahead: Next Steps in Research and Clinical Adoption
Looking forward, several key areas demand focused research to ensure that the promising benefits of giredestrant are fully realized in clinical practice. These include:
- Long-term overall survival data: As more mature data become available, it will be critical to determine the full extent of OS improvement alongside DFS gains.
- Extended safety surveillance: Continued monitoring of adverse reactions will help refine dosage recommendations and support safe long-term use.
- Exploratory studies in different patient populations: Ongoing trials may explore the use of giredestrant in populations with varying risk profiles or in combination with other therapies, further broadening its potential applications.
- Cost-effectiveness assessments: Rigorous studies evaluating the financial impact of adopting giredestrant in standard treatment protocols will be essential to inform reimbursement decisions.
These areas of research are not just academic exercises—they are the next steps in ensuring that breakthroughs in the lab translate into real, tangible benefits for patients. As we continue to figure a path through evolving treatment options, the collaboration between researchers, clinicians, and regulatory bodies remains critical.
Final Thoughts: A New Chapter in Endocrine Therapy?
In conclusion, the phase 3 lidERA trial results shine a spotlight on giredestrant as a potentially transformative treatment for early-stage ER-positive, HER2-negative breast cancer. With significant improvements in invasive DFS, a manageable safety profile, and encouraging early trends in overall survival, the findings invite us to re-examine our current approach to endocrine therapy.
While there remains plenty to figure out—from understanding the small distinctions in patient responses to managing the overlapping challenges of trial adoption—the promise of giredestrant is undeniable. In a field that is often loaded with problems and riddled with tension, it is reassuring to see progress that could translate into longer, healthier lives for patients.
As we work through the puzzle of modern oncology, it is critical that the oncology community continues to engage in open discussion, share real-world experiences, and remain committed to a patient-centered approach. Only by doing so can we truly harness the potential of novel therapies and ultimately steer through the tricky parts of cancer care to usher in a new chapter in treatment.
In the spirit of continuous improvement and dedicated research, giredestrant stands as a testament to the progress made in our understanding of breast cancer biology and the promise held by precision medicine. As further data emerge and real-world applications are tested, it is our hope that this therapy not only improves DFS but also fundamentally alters the trajectory of breast cancer treatment for the better.
Summary Table of Key Points
| Aspect | Details |
|---|---|
| Therapy Type | Oral Selective Estrogen Receptor Degrader (SERD) |
| Trial Name | lidERA (Phase 3) |
| Patient Population | ER-positive, HER2-negative early-stage breast cancer |
| Primary Outcome | Improved invasive disease-free survival (DFS) |
| Safety Profile | Well-tolerated; adverse effects consistent with known endocrine therapies |
| Regulatory Implications | Potential for fast-track designation pending further data |
| Future Research Focus | Long-term OS, combination strategies, cost-effectiveness |
Concluding Remarks
As we reflect on the current state and future implications of giredestrant in early-stage breast cancer, it is clear that both the oncology community and patients stand on the brink of potentially transformative changes in therapeutic strategy. With the balance of promising efficacy and a tolerable safety profile, giredestrant offers a beacon of hope amid the nerve-racking uncertainties often associated with cancer treatment.
The advances outlined here demonstrate that, despite the many complicated pieces and tricky parts of integrating new treatments, progress in modern medicine is indeed possible. By working together and continuing to figure a path through evolving treatment paradigms, the future of endocrine therapy in breast cancer looks not only promising but also genuinely patient-centric.
It is an exciting time in oncology—a time when innovative ideas and new treatments like giredestrant remind us all that even amid tangled issues and challenging decisions, progress can bring about improved patient outcomes and a brighter tomorrow for those affected by breast cancer.
Originally Post From https://www.cancernetwork.com/view/giredestrant-exhibits-dfs-improvement-in-early-stage-breast-cancer
Read more about this topic at
Mayo Clinic discovery of breast cancer treatment …
Inspiring breast cancer breakthroughs