Biomimetic Nanomedicine: A New Chapter in Lung Cancer Treatment
The world of modern medicine is constantly evolving. In my opinion, one of the most promising breakthroughs in the therapeutic arena involves combining the unique benefits of phototherapy with targeted immunotherapy. Recent research has highlighted a novel approach using biomimetic aggregation-induced emission (AIE) luminogens. This innovative platform is designed for enhanced phototherapy and immune checkpoint blockade, demonstrating potential in targeting one of the most challenging aspects of lung cancer treatment.
It is always impressive when scientific progress manages to overcome the traditional hurdles that have bogged down conventional therapies. The work behind these biomimetic AIE agents offers a fresh perspective on how to fight lung cancer using a dual strategy that combines direct tumor ablation with the stimulation of the body’s immune response. In this opinion piece, I will share my views on the potential significance and the road ahead for this technology.
Overcoming the Tricky Parts of Conventional AIEgens
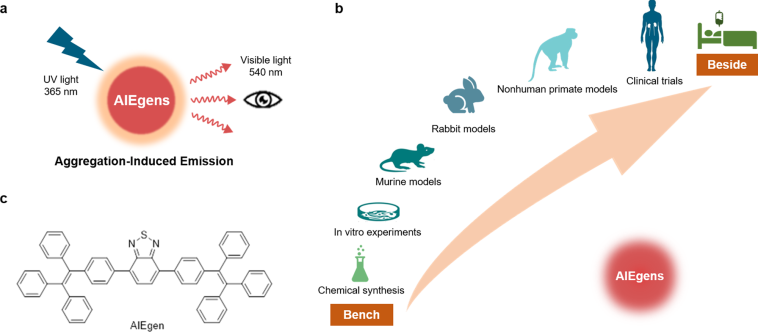
Conventional AIE luminogens have long been recognized for their excellent optical performance. However, many of these agents are burdened with issues such as hydrophobicity, low specificity, and short circulation lifetimes. These obstacles, or as some might call them, the tricky parts and tangled issues, have limited their widespread clinical utility in cancer therapy.
The recent approach involves using a unique photosensitizer, BITT, which is based on an aggregation-induced emission mechanism that not only produces strong fluorescence but also efficiently generates reactive oxygen species (ROS) and converts absorbed light into heat. This multi-functional capacity addresses several of the complicated pieces that have previously hindered the effectiveness of phototherapy techniques in tumor destruction.
Many clinicians and researchers are excited about the possibilities because, by tackling these nerve-racking and overwhelming limitations, this technology opens the door to treatments that are both highly effective and minimally invasive. The combination of BITT’s optical properties with new surface modification techniques has set the stage for improved therapy outcomes.
Enhanced Tumor Targeting with CD8+ T Cell Membrane Coating
One of the most appealing aspects of this novel approach is the utilization of cell membrane coating technology. By camouflaging BITT nanoparticles with CD8+ T cell membranes, researchers have discovered a method to significantly boost their tumor targeting ability. The trick here is that CD8+ T cells naturally recognize and bind to tumor sites through the interaction between PD-1 on the T cell membrane and PD-L1 on cancer cells.
This mechanism acts as a natural homing device for the nanoparticles, ensuring that they accumulate preferentially at the tumor location. As a result, the drug delivery system bypasses many of the confusing bits and twists and turns of conventional nanoparticle delivery methods. In practical terms, this means that the treatment becomes more focused on cancer cells while sparing normal tissues, an outcome that is both clinically appealing and critical for patient safety.
Key Benefits of the Biomimetic Strategy
- Improved tumor accumulation through PD-1/PD-L1 targeting
- Enhanced circulation lifetime compared to uncoated nanoparticles
- Better water compatibility through surface modification
- Simultaneous promotion of phototherapy and immune activation
This innovative tactic addresses many of the subtle parts and fine points that researchers have struggled with, minimizing off-target effects and increasing the therapeutic index.
Photothermal and Photodynamic Therapy: A Combined Approach
The dual functionality of BITT allows it to be an effective agent for both photothermal therapy (PTT) and photodynamic therapy (PDT). In this system, the conversion of light energy into heat (photothermal effect) is complemented by the generation of reactive oxygen species (ROS) for a photodynamic effect. Together, these two modalities work synergistically to kill cancer cells.
The photothermal properties enable the nanoparticles to elevate temperature rapidly, reaching levels (exceeding 50°C) that are sufficient to induce cancer cell apoptosis. Moreover, the production of ROS further enhances the cell-killing effect by damaging cellular components. In my view, this combined approach is a super important step forward as it leverages the strengths of both techniques while compensating for their individual limitations.
Advantages of the Combined Phototherapy Strategy
Consider the following benefits:
- Synergistic Effect: The integration of photothermal and photodynamic effects produces overall greater tumor suppression than either method alone.
- Enhanced ROS Generation: The coating with T cell membranes boosts intracellular ROS, further damaging cancer cells.
- Targeted Heat Production: Local accumulation in tumors results in heat being generated exactly where it is needed.
These bullet points demonstrate that the system not only takes advantage of the natural properties of BITT but also significantly improves upon traditional treatment techniques by mitigating some of the nerve-racking and intimidating challenges encountered in prior research.
Synergistic Immunotherapy: Reinforcing the Body’s Natural Defenses
Alongside phototherapy, the immune system plays a key role in recognizing and eliminating cancer cells. Immunotherapy aims to release the brakes on the body’s own defense mechanisms. The use of biomimetic nanoparticles provides an interesting twist to traditional immunotherapy methods.
The CD8+ T cell membrane not only enhances tumor targeting but also helps to activate an immune response once the nanoparticles reach the tumor microenvironment. This activation is achieved through immune checkpoint blockade, specifically by interfering with the PD-1/PD-L1 interaction. In doing so, the treatment helps to re-stimulate the body’s own T cells, which are essential for a sustained anti-cancer response.
By combining phototherapy with immunotherapy, the approach creates a kind of one-two punch against lung cancer. First, the cancer cells are directly killed via heat and ROS. Then, the immune system is engaged to mop up any residual tumor cells and help prevent recurrence. This combined strategy is loaded with promise and may represent a future direction for integrated cancer treatments.
Immune System Engagement: How It Works
The following bullet points highlight the process:
- Checkpoint Blockade: By targeting PD-1 receptors, the nanoparticles help to block the PD-L1 pathway, making it harder for tumor cells to hide from the immune system.
- T Cell Activation: Increased infiltration of CD4+ and CD8+ T cells in the tumor tissue boosts the overall antitumor immune response.
- Synergistic Effects: The immediate cell-killing by phototherapy is complemented by longer-term immune activation, leading to enhanced outcomes.
This integration of targeted phototherapy with immune activation is an elegant solution to the historically nerve-racking issues associated with conventional cancer treatments.
In Vitro and In Vivo Findings: Real-World Implications
Recent experimental studies have provided some solid evidence of the potential benefits of these biomimetic AIE nanoparticles in lung cancer therapy. Laboratory-based cell studies (in vitro) have shown that the nanoparticles effectively bind to lung cancer cells in a dose- and time-dependent manner. This binding is largely due to the PD-1/PD-L1 interaction, which serves as a natural targeting mechanism.
Furthermore, by using laser irradiation, the BITT and its cloaked counterpart were proven to generate significant heat and ROS, leading to successful tumor cell death. These promising findings were then corroborated in animal models (in vivo), where the nanoparticles exhibited excellent tumor accumulation. In addition, the circulation lifetime of the CD8+ T cell-coated BITT was significantly prolonged compared to its uncoated version, which is critical for effective treatment delivery.
Table: Comparison of Key Parameters
| Parameter | BITT (Uncoated) | TB (CD8+ T Membrane Coated) |
|---|---|---|
| Hydrodynamic Diameter (nm) | ~130 | ~200 |
| Zeta Potential (mV) | -15 | -30 |
| Tumor Accumulation | Moderate | High (via PD-1/PD-L1 targeting) |
| Photothermal Effect (Temp Rise) | Significant under irradiation | Enhanced (reaching ~58°C) |
| Circulation Lifetime | ~5 hours | ~8 hours |
This table helps to clarify the advantages of applying a biomimetic coating to the BITT nanoparticles. It shows that the enhancements are not just theoretical but translate into measurable improvements that could change how lung cancer is managed.
Addressing the Confusing Bits of Biosafety and Side Effects
One of the greatest challenges when introducing any new therapeutic agent is ensuring that it is safe for patients. The studies on these biomimetic nanoparticles have included extensive biosafety evaluations in animal models. While many may find the toxicology data from preclinical studies somewhat intimidating and loaded with problems, the results have been promising so far.
For instance, the body weight of test subjects remained stable throughout treatment, and routine blood tests showed no significant deviations from normal parameters. Moreover, histological examinations of major organs, such as the heart, liver, spleen, lungs, and kidneys, did not reveal any organ damage or inflammatory lesions.
These outcomes suggest that when used with precision, the treatment has a minimal side effect profile. In today’s landscape of targeted therapies, the side effects of traditional therapies are being widely re-evaluated. Such innovative technologies are providing an alternate pathway that might steer through the intimidating maze of issues related to toxicity.
Bullet List: Key Biosafety Observations
- Stable body weight in treated subjects
- Normal blood parameters and routine hematology
- No significant histological damage in major organs
- Prolonged blood circulation allowing sustained action
These observations are particularly encouraging in the context of lung cancer, a disease that often requires treatments which are super important to be both effective and safe. It is critical to keep in mind that while preclinical data is promising, further clinical investigations will need to confirm these safety profiles in human systems.
Future Directions: From Lab Bench to Clinical Bedside
While the current findings are extremely promising, there are still several confusing bits and challenging pieces that require further study before this technology is translated into a clinical setting. The path ahead involves addressing issues such as long-term nanoplatform stability, storage conditions, and comprehensive toxicology profiles in human subjects.
There is also the question of the long-term immunotherapeutic efficacy. Some experts suggest that future studies should take a closer look at how the nanoplatform affects memory T cell subsets, such as central memory T cells. Understanding these fine shades of immune modulation will be super important for harnessing a sustained therapeutic effect and preventing recurrence of the disease.
Moving forward, I believe that combining robust nanotechnology engineering with precise biochemical targeting will be critical. Researchers and clinicians must work hand in hand to figure a path that fills in the gaps in our current understanding. Collaborative efforts between nanotechnologists, immunologists, and clinical oncologists will be key to overcoming any remaining tangled issues.
Short-Term and Long-Term Challenges
Some potential hurdles include:
- Stability Under Storage: Ensuring that the nanoparticles remain effective over long periods and under various storage conditions.
- Comprehensive Toxicology Studies: Conducting extensive safety studies to rule out any hidden complexities that could result in adverse outcomes when translated into humans.
- Clinical Translation: Designing and executing carefully structured clinical trials that assess both the immediate therapeutic outcomes and long-term immune responses.
- Manufacturing and Scalability: Developing cost-effective methods for manufacturing these complex nanostructures while maintaining quality and consistency.
Addressing these bullet points in a methodical manner will not only help refine the technology but also build the confidence of regulatory bodies and clinicians alike.
Integrating Phototherapy with Personalized Medicine
One of the most appealing prospects of this technology is its potential as a cornerstone of personalized cancer treatments. With the push towards individualized care, the ability to design therapies that are tailored to the patient’s own biology is becoming increasingly important. The biomimetic approach truly exemplifies this trend by using components derived directly from T cells to enhance delivery and targeting.
Personalized medicine requires that we consider every patient’s unique set of conditions, and the integration of phototherapy with immunotherapy may offer the flexibility to adjust treatment parameters on a per-patient basis. With well-controlled light irradiation and customizable nanoparticle designs, clinicians may soon be able to manage the fine details of treatment protocols with unprecedented precision.
Key Advantages in a Personalized Setting
- Target Specificity: The use of T cell membranes provides an innate mechanism to target tumor cells more precisely, reducing off-target effects.
- Controlled Treatment Delivery: The ability to activate the nanoparticles using an external light source allows for controlled timing and dosage.
- Immune Stimulation: The reactivation of the patient’s own T cells ensures that the therapy is not only direct but also leads to long-term immune surveillance.
- Reduced Side Effects: With localized therapy and targeted delivery, there is potential for reduced systemic toxicity.
These factors all contribute to a treatment paradigm that is as safe as it is effective. The benefits of such a personalized approach could ultimately lead to more positive outcomes for lung cancer patients, offering them hope where conventional therapies may have fallen short.
Balancing Innovation with Clinical Practicality
The fast-paced evolution of nanomedicine raises exciting possibilities but also demands that we consider practical issues related to clinical adoption. For a technology to be translated from the lab bench to the clinical bedside, it must be not only effective but also feasible in terms of manufacturing, regulatory approval, and cost-effectiveness.
Current research using TB nanoparticles has provided a strong proof-of-concept. The synthesis protocols are relatively straightforward, involving self-assembly and a series of physical processes such as sonication and extrusion. However, every innovative method is bound to confront the challenging parts that come with scaling up production and ensuring reproducibility in a clinical environment.
Practical Considerations Listed
- Scalability: Can the synthesis methods be reliably reproduced on a large scale?
- Quality Control: How can we ensure that every batch of nanoparticles meets stringent purity and efficacy standards?
- Regulatory Challenges: What will be the hurdles during clinical approval, especially regarding long-term safety?
- Cost-Effectiveness: Can the final product be provided at an affordable cost without compromising quality?
In my view, addressing these practical concerns will be as important as demonstrating cutting-edge efficacy in research. Both researchers and regulators must work together to steer through the intimidating maze of clinical translation to ensure these promising therapies reach the patients who need them most.
Reflections on the Future of Lung Cancer Therapy
The integration of biomimetic AIE luminogens with targeted immune therapies represents a revolutionary shift in lung cancer treatment. It is exciting to watch how the field is progressing to combine multiple therapeutic modalities into one unified strategy. This new modality does more than simply kill tumor cells; it also rallies the patient’s own immune system to combat the disease long after the initial treatment.
While there are still several confusing bits and challenging pieces to sort out, the collective research so far suggests that this approach could be a turning point for patients with aggressive lung cancer. The ability to simultaneously deliver intense photothermal effects and stimulate immune responses represents an adaptable strategy in a field that has been searching for more reliable, less toxic alternatives.
In addition, the use of natural cell membranes as a component of these nanoplatforms highlights the potential for a more biocompatible, less off-putting approach to cancer therapy. As researchers continue to optimize the surface characteristics of these nanoparticles, we can expect even greater specificity and fewer side effects, making the treatment not only effective but also patient-friendly.
Future Research Priorities
Looking forward, I believe that the following areas are key for future research:
- Long-Term Efficacy Studies: Focus on understanding how these treatments perform over extended periods, particularly regarding immune memory and cancer recurrence.
- Detailed Toxicological Analysis: Thoroughly evaluate safety profiles in more diverse preclinical and clinical settings to ensure minimal side effects.
- Enhanced Delivery Strategies: Continue to explore and refine the methods of nanoparticle delivery and targeting to further increase tumor specificity.
- Combination Therapies: Investigate how these nanoparticles might work in tandem with other therapeutic strategies such as chemotherapy or targeted small molecule drugs.
Prioritizing these research avenues could help overcome the nerve-racking and overwhelming challenges that sometimes hold back new therapies from reaching clinical practice. With continued innovation and collaboration among scientists, clinicians, and regulatory agencies, the dream of more effective lung cancer treatments could soon become a reality.
Concluding Thoughts: A Promising Path Forward
In summary, the development of biomimetic AIE nanoparticles coated with CD8+ T cell membranes is an exciting example of how modern medicine can integrate advanced nanotechnology with targeted immunotherapy. This dual-action system not only offers a way to enhance phototherapy by overcoming the tricky parts of conventional methods but also reactivates the body’s natural defenses against lung cancer.
The synergistic approach, combining both photothermal/photodynamic effects and immune checkpoint blockade, represents a fresh and promising pathway for treating a disease that has historically been resistant to many conventional therapies. With encouraging results from both in vitro and in vivo studies, this technology is poised to pave the way for more personalized and effective lung cancer treatments.
While some of the hidden complexities and intimidating challenges still need to be addressed, the current data gives reason to remain optimistic. The journey from early research findings to clinical practice is never straightforward – it is filled with confusing bits, tangled issues, and a few nerve-racking hurdles. Nevertheless, the innovative use of natural cell membranes to boost targeting, together with potent phototherapeutic effects, provides a similar level of promise that could reshape how we approach lung cancer therapy in the near future.
Key Takeaways
- The use of CD8+ T cell membranes to coat AIE luminogens improves targeting to lung tumors through natural PD-1/PD-L1 interactions.
- The dual action of photothermal and photodynamic therapy offers a potent cell-killing mechanism that leverages both heat and ROS generation.
- In vitro and in vivo studies have shown significant improvements in tumor accumulation, prolonged circulation time, and increased therapeutic efficacy.
- The integration of phototherapy with immune checkpoint blockade represents a super important advancement in combining direct and immune-mediated cancer treatment strategies.
- Safety studies to date are encouraging, though further research will be necessary to address storage, scalability, and long-term effects.
Looking ahead, it is essential to continue research on these biomimetic platforms, not only to optimize their design and functionality but also to explore their integration into broader treatment regimens that may combine other targeted therapies. This kind of multidisciplinary approach will likely be the key to successfully managing and eventually overcoming aggressive lung cancers.
Final Reflections
As we take a closer look at the ever-evolving field of lung cancer therapy, it becomes clear that innovative treatments like biomimetic AIE luminogens have the potential to usher in a new era. Although there are still several challenging parts and a few overwelming issues to work through, the combination of phototherapy and immunotherapy offers a highly promising strategy for patients who have long awaited more effective treatments.
This technology, with its precise targeting, minimal side effects, and enhanced therapeutic outcomes, represents a promising step forward. It encourages us to continue investing in advanced nanotechnology and immunotherapy research while remaining aware of the practical challenges that lie ahead. With ongoing collaboration and dedication, these cutting-edge approaches may soon transition into routine clinical practice, providing hope and improved lives for many patients.
Ultimately, the journey from innovative research to everyday clinical application is one filled with twists and turns, requiring resilience and creativity. In my view, the advances presented by biomimetic AIE nanoparticles highlight a future where personalized, integrated cancer therapies not only become feasible but also herald a new standard of care in oncological treatments.
In conclusion, while further studies are needed to fully fine-tune this promising approach, the integration of sophisticated nanomedicine into lung cancer therapy is a testament to the progress that is possible when science dares to tackle the tricky and intimidating challenges of modern medicine. I remain hopeful that, in the not-too-distant future, these advancements will lead to safer, more effective treatments and a renewed sense of optimism for lung cancer patients worldwide.
Originally Post From https://www.dovepress.com/biomimetic-aggregation-induced-emission-luminogens-mediated-effective–peer-reviewed-fulltext-article-IJN
Read more about this topic at
Cell membrane-based biomimetic technology for cancer …
Cell membrane-based biomimetic technology for cancer …