Examining the Enigma of Myelodysplastic Syndrome
The battle against blood cancers has long been fraught with challenges that many describe as a game of whack-a-mole. Researchers are now working through the tangled issues of myelodysplastic syndrome (MDS), a form of blood cancer known for its tricky parts and complicated pieces. As a group of experts in the field, including those from the University of Colorado Cancer Center, have shown, there is a pressing need for new treatment strategies that go beyond existing options. In this opinion piece, we take a closer look at the ongoing journey from the laboratory bench to the patient’s bedside and back again—a process that highlights both the promise and the pitfalls of current research into MDS.
Understanding MDS and Its Tricky Nature
MDS is not a single disease but a spectrum of blood disorders where the body’s ability to produce healthy blood cells is severely impaired. This results in a cascade of problems, including poor immune function, impaired oxygen transport, and problems with blood clotting. Many describe the process of understanding MDS as digging into a labyrinth of tangled molecular and genetic issues—with every twist and turn revealing new challenges. Unlike more straightforward conditions, MDS comes with a series of tricky parts that require innovative approaches to decipher and ultimately treat.
What Makes MDS So Challenging?
The tricky parts of MDS lie in its diversity. The disease is loaded with issues, with each patient often presenting with a unique set of genetic mutations. These little details—the fine points that vary from case to case—make one-size-fits-all treatments almost impossible. Researchers are finding that the malignant blood-forming stem cells in MDS are not uniform; they are a moving target, adapting and evolving as the disease progresses. This creates a nerve-racking situation for clinicians and scientists alike as they work to figure a path through the maze of genetic variability.
Key Observations from Recent Studies
Current research into MDS is driven by the need to identify unique biological markers and vulnerabilities within these malignant stem cells. Insights gained from previous clinical trials demonstrate that while some drug combinations yield improvements for certain patients, others continue to defy conventional treatments. For instance, studies leveraging a combination of omacetaxine—a drug that interferes with protein synthesis—with standard therapy have led to some encouraging results. However, the disease’s ability to adapt remains a sticking point. As one researcher put it, treating MDS can feel like trying to stop a series of moles from popping up—all while the very cells under investigation shift their behavior and identity.
From Laboratory Bench to Patient Bedside—And Back Again
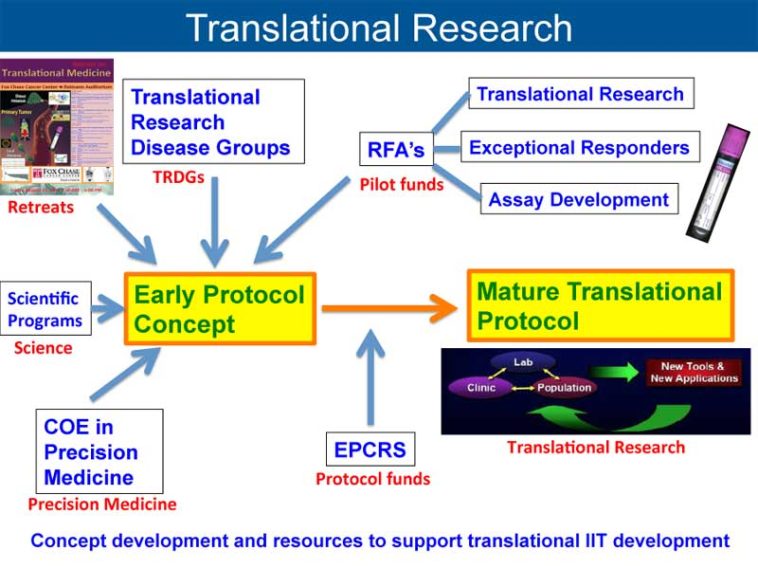
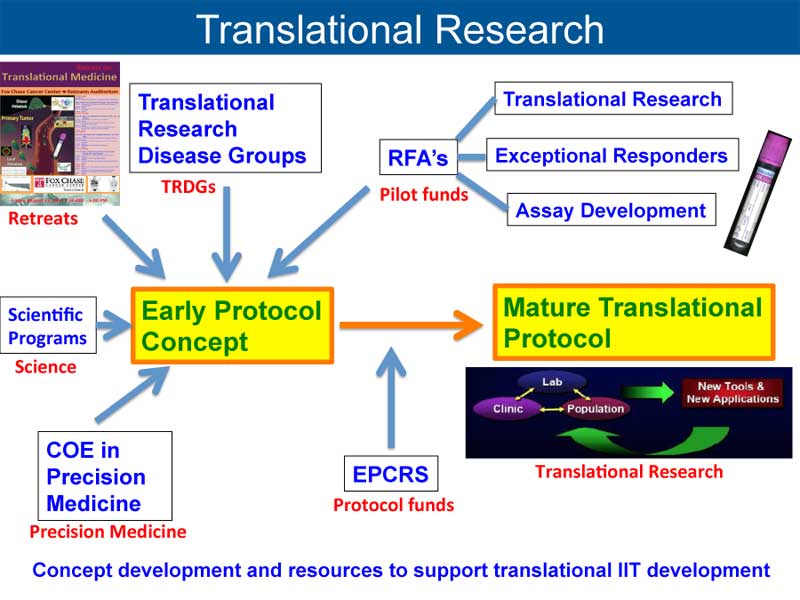
It is this bed-to-bench loop that is redefining how the scientific community is approaching MDS research. The concept of “bench to bedside to bench” encapsulates a continual process of learning and adaptation. The journey starts with laboratory discoveries that are tested in clinical settings; the outcomes of these trials then loop back into the research phase, helping scientists dig into what worked, what didn’t, and why. In this dynamic process, both preclinical and clinical studies inform each other, creating a roadmap for the development of more effective therapies.
Collaboration and Shared Resources: The Backbone of Research
The collaborative efforts at institutions such as the CU Cancer Center have been critical in developing new therapeutic avenues. By pooling resources like cell sequencing technology and advanced flow cytometry, researchers can sort out the fine shades and subtle details of MDS stem cell behavior. This cooperative model not only strengthens the research but also speeds up the translation of new discoveries into clinical trials. The smooth interplay between research and clinical teams is an essential factor in driving breakthroughs forward.
Scholarly and Clinical Partnerships in Action
An exemplary demonstration of this collaborative spirit was seen in a clinical trial led by CU hematologist Dan Pollyea, where patients with high-risk MDS received a combination therapy. When patients responded well to the treatment, it provided a tangible proof-of-concept. Yet, for others, the disease persisted. These differences have prompted researchers to dig into the nitty-gritty of malignant stem cell properties, setting the stage for a new round of exploration into tailored therapeutic strategies.
Targeting the Malignant Stem Cells: A Whac-A-Mole Dilemma
One of the biggest hurdles in treating MDS is its resistance to therapies aimed at its stem cells. Many describe this situation as a game where intervention against one problematic cell only sees another popping up—the equivalent of a never-ending game of whack-a-mole. The malignancies in these stem cells do not behave uniformly, which means that while one treatment might knock out one set of cells, another group, with different hidden complexities, might survive. This phenomenon is both challenging and overwhelming, making researchers work tirelessly to define the unique characteristics of each group of cells.
Innovative Strategies for a Multifaceted Problem
In response to this challenge, scientists are now researching ways to categorize MDS stem cells based on their unique biological signatures. Here are some of the approaches currently being explored:
- Subtype Identification: Researchers are using advanced genetic profiling techniques to sort out different MDS subtypes by identifying small distinctions in their genetic makeup.
- Tailored Therapeutics: Once a subtype is defined, therapies can be designed to target its specific vulnerabilities, aiming to outmaneuver the cancer’s crafty nature.
- Combination Therapies: By combining drugs that work through different mechanisms, clinicians hope to hit multiple targets at once, reducing the chances of the disease adapting and surviving treatment.
A table summarizing the treatment approaches may help clarify these strategies:
| Approach | Description | Challenges |
|---|---|---|
| Genetic Profiling | Sorting MDS based on unique genetic mutations | High variability; technical demands |
| Tailored Therapeutics | Designing drugs specific to cell subtype vulnerabilities | Requires in-depth molecular knowledge |
| Combination Therapies | Using multi-drug approaches to target several cell types simultaneously | Managing side effects and drug interactions |
Clinical Trial Lessons: What Works and What Doesn’t
Every clinical trial offers a wealth of learning opportunities—even when the immediate outcomes are less than ideal. With nearly 400 clinical trials conducted over the past two decades in the field of MDS alone, it is clear that progress has been slow and the road ahead remains intimidating. Among these efforts, the recent trial involving omacetaxine plus azacitidine stood out. While some patients showed marked improvement, others continued to struggle. This divergence in results has raised crucial questions about the inherent nature of MDS and the robustness of our current treatment arsenals.
Insights from the Omacetaxine-Azacitidine Study
The combined approach of using omacetaxine with a standard treatment like azacitidine has provided mixed outcomes. Although a significant number of newly diagnosed patients benefited, a sizable group showed disease progression despite the treatment. This variation underscores the need to understand the nuanced behavior of the malignant stem cells that persist in the face of therapy. Researchers have taken this as a cue to concentrate more on the biology of non-responsive cells in order to pave the way for new, more effective drug combinations.
What Patients and Physicians Can Learn
The lessons emerging from these clinical trials are invaluable. They serve as a reminder that even when one treatment shows promise, there is often a subgroup of patients for whom the battle is far from over. For health professionals, these insights encourage an ongoing dialogue between the lab and the clinic—a dialogue that is essential for continuously refining treatment protocols. For patients, the message is one of cautious optimism: while current treatments may be effective for some, the search for more universally successful therapies is well underway.
Unraveling the Hidden Complexities of MDS Stem Cells
The journey to better treatments involves getting into the fine details of what makes each group of MDS stem cells tick. Research is increasingly focused on the unique, hidden complexities that define why certain stem cells are resistant to therapies—a crucial step for developing strategies that can more effectively target these resilient cells.
Exploring Genetic Markers and Mutations
One promising avenue involves the detailed study of genetic markers that reveal the underlying tendencies of MDS stem cells. By categorizing these subtypes based on the specific mutations they harbor, scientists are tailoring their approaches to each subgroup’s needs. For example, some stem cells demonstrate vulnerabilities in protein synthesis processes, which has spurred interest in drugs that inhibit these pathways. This precise form of molecular mapping enables researchers to design therapies that have a better chance of overcoming the defense mechanisms those cells employ.
Mechanistic Studies: The Roadmap to Personalized Medicine
Mechanistic studies—those that take a closer look at the small distinctions within the cell’s operational machinery—are currently at the forefront of MDS research. These studies dive into the subtle parts of cell biology to uncover how certain mutations might protect malignant cells from standard therapies. In doing so, scientists hope to turn around the current paradigm, moving from a generalized treatment model to one that is personalized and highly targeted. The overall goal is to reduce the persistent bad actor cells and shift the treatment landscape from a broad-spectrum approach to one that exploits the unique vulnerabilities of each cell group.
The Promise of Combination Therapies
Given the multifaceted nature of MDS and its tendency to evade singular treatment strategies, a growing consensus within the research community supports the use of combination therapies. These regimens, which often pair new experimental agents with established treatments, aim to attack the disease from multiple angles simultaneously. This strategy is not without its tricky parts—balancing drug interactions and managing side effects can be overwhelming—but the potential for synergistic effects has generated significant excitement.
Advantages of Multi-Agent Therapy
When working through a multifaceted disease like MDS, a combination of therapeutic agents may provide the following advantages:
- Broader Reach: By deploying drugs that act on different cellular processes, clinicians can increase the likelihood of hitting resistant cell populations.
- Delayed Resistance: Using multiple agents reduces the chance that malignant cells will adapt quickly, a significant risk with monotherapy.
- Enhanced Efficacy: Early data suggest that some combinations may yield improvements that cannot be achieved by single-agent treatments alone.
Challenges in Implementing Combination Regimens
Despite the promise, combination therapies come with their own set of tricky parts. Researchers must be particularly cautious about the following issues:
- Adverse Interactions: Drugs may interact in unexpected ways, leading to side effects that can be both off-putting and dangerous.
- Dosing Complexity: Striking the right balance and scheduling of doses to maximize effectiveness while minimizing toxicity can be nerve-racking.
- Individual Variability: The variability among patients means that what works for one subgroup might not be suitable for another, further complicating treatment plans.
Bridging the Gap Between Research and Clinical Practice
The notion of a seamless connection between fundamental research and clinical practice is at the core of modern medicine. With the evolving understanding of MDS, clinicians and researchers are increasingly working side by side to turn laboratory discoveries into real-world treatments. This collaborative, iterative process is essential for transforming innovative ideas into therapies that genuinely improve patient outcomes.
Integrating Laboratory Discoveries with Patient Care
At institutions like the CU Cancer Center, integration is not just a buzzword—it is the everyday reality. The synergy between clinicians and researchers has allowed for rapid feedback loops, where treatment successes and failures inform ongoing experiments. This method of taking a clinical observation, then reexamining the underlying biology in the lab, and vice versa, is proving to be a promising model for accelerating advances in MDS treatment.
Key Strategic Elements for Successful Integration
For a seamless connection between the lab and the clinic, several key elements are essential:
- Open Communication: Constant collaboration and dialogue between clinical practitioners and laboratory scientists ensure that insights are shared in real time.
- Resource Sharing: Access to advanced technological resources such as cell sequencing and detailed flow cytometry is critical for in-depth analysis.
- Flexibility in Research Design: Being able to pivot quickly based on clinical feedback allows researchers to adjust their hypotheses and experimental designs.
- Patient-Centric Focus: Ultimately, the integration is aimed at improving outcomes for patients, ensuring that each discovery has practical therapeutic applications.
Looking at the Future: Emerging Therapies and Ongoing Trials
While MDS continues to pose daunting challenges, the future is brimming with potential breakthroughs. Researchers are now testing new therapies that specifically target the problematic stem cells, harnessing the power of both traditional and innovative approaches. This new wave of clinical trials is more informed than ever by detailed bench research that picks apart the little twists in MDS biology.
Emerging Agents and Their Potential Impacts
A number of experimental agents are showing promise in preclinical tests. Key emerging strategies include:
- Novel Protein Synthesis Inhibitors: These agents are designed to disrupt the cellular machinery in malignant stem cells, potentially thwarting their ability to thrive.
- Targeted Molecular Inhibitors: By focusing on specific genetic mutations, these inhibitors aim to neutralize the bad actors within the MDS cell population.
- Immunomodulatory Approaches: In addition to directly attacking cancer cells, some therapies are geared toward empowering the patient’s immune system to fight back more effectively.
Assessing the Clinical Trial Landscape
The high number of clinical trials conducted over the past decades has shown that the field is far from stagnant. In fact, the accumulation of data from these trials provides a rich resource from which new hypotheses can be formed. For example, the almost 400 clinical trials in the MDS arena reveal a clear need for strategies that are not only effective in isolating malignant cells but are also capable of addressing the varying genetic landscapes that exist within patient populations.
Despite the overwhelming challenges, each new study offers insights that guide researchers toward more refined, targeted therapies. This iterative process, circling from lab to clinic and back, represents a dynamic approach that is particularly important for a disease as variable and stubborn as MDS.
Taking the Wheel: The Role of Personalized Medicine in MDS
As we work through the fine points of MDS research, one conclusion becomes increasingly clear: the future of treatment lies in personalized medicine. No two cases of MDS are exactly alike, and the one-size-fits-all approach is often insufficient. Instead, clinicians and researchers are now aiming to design therapies that are uniquely tailored to the specific characteristics of each patient’s disease.
Personalization in Practice: A Closer Look
Personalized medicine has several key advantages, particularly in the context of a disease as multifaceted as MDS:
- Improved Treatment Outcomes: By targeting the unique vulnerabilities of different MDS subtypes, personalized medicine holds the promise of more effective interventions.
- Reduced Side Effects: Tailoring treatment to individual profiles can help minimize adverse reactions often seen with broader-acting therapies.
- Enhanced Patient Satisfaction: Customized treatment plans can make patients feel more involved in their own healthcare decisions, ultimately boosting confidence and compliance.
Strategies for Implementing Personalized Therapies
Several strategies are being developed to incorporate personalized techniques into the treatment of MDS:
- Genomic Profiling: Using comprehensive genetic analyses to pinpoint the exact mutations and variations present in a patient’s malignant cells.
- Biomarker Development: Identifying the small distinctions that can serve as reliable indicators for treatment sensitivity or resistance.
- Adaptive Clinical Trials: Designing studies that can adjust the treatment protocol in real time based on interim patient responses and emerging data.
The Broader Implications for Blood Cancer Research
The in-depth exploration into MDS is not taking place in isolation. The methodologies, collaborations, and discoveries from this research effort have broader implications for the treatment of other blood cancers. Lessons learned from the fine details of MDS stem cell behavior are informing studies in acute myelogenous leukemia and other related disorders.
Shared Challenges Across Blood Cancers
While each blood cancer has its own set of confusing bits and challenging pieces, many share common hurdles:
- Diverse Genetic Profiles: Like MDS, other blood cancers often present with varied genetic alterations that can make targeted treatment difficult.
- Tumor Evolution: Malignant cells in many blood cancers adapt over time, rendering some therapies less effective as the disease evolves.
- Resistance Mechanisms: Whether in MDS or other blood cancers, the ability of cancer stem cells to resist intervention remains one of the most daunting challenges for researchers.
Advancing the Field Through Shared Innovations
Innovations born out of the battle against MDS—such as refined genomic profiling techniques and adaptive clinical trial designs—are now being extended to other cancers. This cross-pollination of ideas benefits the overall field of oncology by creating a more flexible and informed approach to treatment. Researchers are increasingly optimistic that the insights gained from MDS research will help steer through similar challenges encountered in other blood cancer subtypes.
Patient Perspectives and the Role of Hope
Amid the scientific rigor and technical discussions, it is important to remember that behind every clinical trial and laboratory experiment, there are patients whose lives are deeply impacted by these diseases. For many, the current landscape of MDS treatment—while showing small incremental improvements—is still filled with overwhelming uncertainty. The progress reported in recent studies, although sometimes measured in small steps, provides essential hope and a reason to press forward.
Understanding Patient Experiences
Patients with MDS often face a cascade of challenges that extend beyond clinical symptoms. The uncertain nature of the disease, the fear of its progression to conditions such as acute myelogenous leukemia, and the constant need for medical interventions contribute to an emotionally charged journey. For these patients, the promise of more personalized and effective treatments is not merely about statistics—it is about reclaiming their quality of life and their ability to plan for the future.
The Emotional and Psychological Impact
The psychological toll of living with a disease loaded with issues like MDS should not be understated. On one hand, continuous research offers hope, while on the other, the relentless thrust of the disease can feel overwhelming. The integration of mental health support alongside advances in medical treatment is a key element in managing the overall wellbeing of patients. Psychological support, teamed with cutting-edge therapies, can help ease some of the nerve-racking uncertainty that accompanies a diagnosis of MDS.
Real-World Challenges: Implementing New Therapies
As promising new therapies emerge from the research lab, the next step is to successfully implement these treatments in everyday clinical practice. This transition is not straightforward; it involves a series of procedural, regulatory, and logistical challenges. Healthcare providers must deal with the twisted issues of drug approval, insurance coverage, and the constant need for patient education regarding new treatment protocols.
Regulatory and Logistical Hurdles
Before any new therapy can become widely available, it must pass rigorous clinical trials and receive approval from regulatory bodies. This process, while essential for ensuring patient safety, can be intimidating and off-putting. The journey from a promising laboratory discovery to a fully approved drug is long and often marked by setbacks. Researchers and clinicians must work through each step with precision, ensuring that every new therapy meets the strict criteria required for safe human use.
Strategies for Streamlining Adoption
Several strategies are being explored to make the adoption of new MDS therapies smoother:
- Enhanced Communication: Better integration between regulatory agencies, researchers, and clinicians can help expedite the review process.
- Pilot Programs: Early adoption programs and pilot studies in select medical centers can provide valuable real-world data that supports broader use.
- Patient Advocacy: Engaging with patient groups to advocate for faster access to innovative treatments ensures that patient voices remain a central part of the process.
The Path Forward: Embracing Innovation and Resilience
Despite the tangled issues and confusing bits that come with treating MDS, the future holds a wealth of opportunity. The ongoing research represents a commitment to finding better ways to target malignant blood-forming stem cells—a commitment that is as much about scientific curiosity as it is about patient care. As we take a closer look at the evolving treatment landscape, it becomes clear that every challenge faced offers a stepping stone toward new innovations.
Optimism Rooted in Science
The current momentum in MDS research is driven by both perseverance and ingenuity. Each setback encountered in the lab has provided valuable insights that help shape the next phase of investigations. Experts remain optimistic that with every refined approach and every adaptive clinical trial, we are gradually turning the tide against a group of cancers that have long defied simple solutions.
Emphasizing a Patient-Centered Approach
At the heart of this research journey lies a commitment to patient care. The goal is not merely to add another drug to the market but to offer hope, dignity, and improved outcomes to those living with MDS. By emphasizing personalized therapies, robust patient support systems, and an unwavering dedication to scientific discovery, the healthcare community is steadily making progress in satisfying the critical need for more effective treatments.
Conclusion: Reflecting on the Journey and Charting the Future
The multi-year quest for better treatments for MDS underscores the intrinsic challenges of modern oncology. The journey from the laboratory bench to the patient’s bedside—and the inevitable return to the bench—illustrates the evolving complexity of battling a disease that is as adaptive as it is deadly. While the road remains loaded with issues and every step presents its own intimidating challenges, the progress made so far gives reason to be hopeful.
Ultimately, the fight against MDS is about more than just research; it is about reclaiming lives. With collaborative efforts that integrate cutting-edge technology, tailored therapeutic strategies, and a deep-rooted patient focus, the future of MDS treatment is on a promising path. The fine details of research may change the way we understand and treat other blood cancers as well, proving that every discovery brings us one step closer to turning the tide in the battle against these stubborn malignancies.
In my view, as we work through the many layered challenges, it is crucial for the medical community to continue investing in both basic and clinical research. It is through the perseverance of scientists who are willing to dig into the nitty-gritty, and the courage of patients who endure and collaborate, that we can hope to transform today’s experimental treatment into tomorrow’s standard-of-care.
Every study, every trial, and every innovative drug represents a collective stride toward easing the nerve-racking uncertainty associated with MDS. As we continue to map out the subtle parts and hidden complexities of these blood cancers, we also weave a narrative of resilience, hope, and unstoppable human determination. The research may sometimes feel like an endless game of whack-a-mole, yet each whack brings us nearer to the day when we can finally claim: we have outsmarted this elusive foe.
As we look to the future, the integration of diverse research techniques, the promise of personalized medicine, and the tireless spirit of collaboration within the scientific community will be our guiding lights. By embracing these challenges and vital new insights, we are not only finding our way through the maze of modern oncology but are also opening avenues for more comprehensive care that extends beyond MDS to a broader spectrum of blood cancers.
In summary, while the journey is far from over and the path is as complicated as it is promising, the dedication of researchers and clinicians alike continues to inspire hope and reshape our understanding of MDS. The battle is ongoing, but with every trial and every refined strategy, we move closer to new therapeutic horizons that might one day turn this once overwhelming challenge into a manageable condition—and ultimately, a conquered adversary.
Originally Post From https://news.cuanschutz.edu/cancer-center/pietras-stevens-myelodysplastic-syndrome
Read more about this topic at
Translational Cancer Research: Home
Translational Research