AAV Gene Therapy for Ovarian Cancer: A New Frontier in a Complex Landscape
Ovarian cancer continues to be one of the most challenging diseases in women’s health. Although treatments such as surgery and chemotherapy have seen considerable improvements over the years, the disease still remains full of problems—often leading to disappointing long-term outcomes. As researchers and clinicians work to figure a path through the tangled issues of treatment resistance and tumor recurrence, a promising new approach has emerged: gene therapy using adeno‐associated virus (AAV) vectors. In this op‐ed, we take a closer look at the potential of AAV gene therapy for ovarian cancer, exploring its key aspects, hurdles, and future directions in a detailed manner.
Introducing the Concept of AAV Gene Therapy
The idea behind gene therapy is to deliver therapeutic genes into cells to correct or modulate disease functions. In the case of ovarian cancer, researchers are trying to make use of AAV vectors to insert genes that can suppress tumor growth, inhibit angiogenesis (the process by which tumors build their own blood vessels), or stimulate the body’s immune response against cancer cells. Compared to other viral vectors, AAV provides several advantages: it is relatively safe, has low immunogenicity, and can enable long-term gene expression. All these characteristics make AAV a super important candidate for targeting ovarian cancer, despite the many tricky parts associated with gene delivery.
Why Ovarian Cancer Demands Innovative Approaches
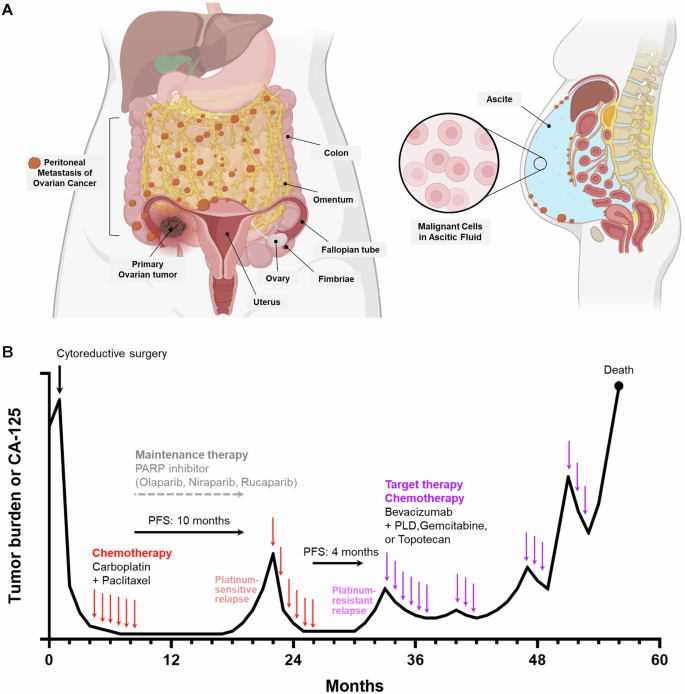
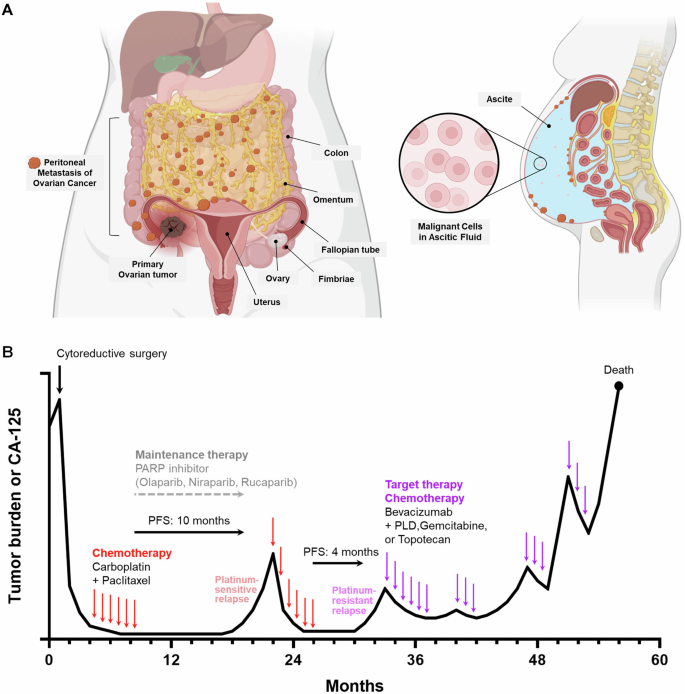
One of the most overwhelming factors in ovarian cancer is the late detection of the disease. Due to its subtle symptoms and confusing bits in its early stages, ovarian cancer is often diagnosed when it has already spread beyond the ovaries. As a result:
- Standard treatments become much less effective.
- Cancer cells develop resistance to the drugs used in chemotherapy.
- The tumor microenvironment becomes heavily loaded with factors that promote tumor growth.
This situation calls for a reevaluation of the treatment strategies. With gene therapy, doctors hope to target not just the cancer cells directly but also to influence the surrounding tumor environment. This approach offers promise in extending progression-free survival and, possibly, even achieving a more durable remission.
Key Advantages of AAV Vectors in Cancer Treatment
AAV vectors have several advantages that make them attractive for gene therapy. They are capable of delivering genes into both dividing and non-dividing cells, a quality that is essential when trying to target cancer cells that may be in a dormant or slow-growing state. Some essential advantages include:
- Low Immunogenicity: Because AAV does not provoke a strong immune response, it avoids the early clearance that plagues some other viral vectors.
- Long-Term Expression: AAV vectors can provide prolonged gene expression, which is a key feature for sustaining a therapeutic effect over extended periods.
- Safety Profile: After years of research and multiple clinical trials, the safety of AAV vectors in humans has been confirmed for several applications.
Understanding the Tricky Parts of Ovarian Cancer Gene Therapy
The challenges in applying gene therapy to ovarian cancer are many. One of the fundamental issues is the low efficiency of targeting every cancer cell. Since ovarian tumors can develop in bulky masses with a chaotic blood supply, delivering the therapeutic gene throughout the entire tumor remains a nerve-racking challenge. Some of the notable difficulties include:
- Targeting Efficiency: It is extremely difficult to ensure that 100% of tumor cells receive the therapeutic gene. Even if a portion of the tumor is transduced, clonal expansion of the untreated cells can eventually lead to relapse.
- Tumor Heterogeneity: Ovarian cancers are known for their tangled issues related to intra-tumoral heterogeneity. This means that within one tumor, there can be many subpopulations of cells, each with slightly different behaviors and genetic profiles. This variety can lead to unpredictable responses.
- Delivery Routes: Researchers must determine the most effective way to deliver AAV vectors. The choice between intravenous (IV) and intraperitoneal (IP) administration can significantly influence the amount of vector that reaches the tumor sites.
Engineering AAV for Greater Specificity
One of the most exciting advances in recent years has been the ability to engineer AAV capsids for improved specificity. By altering the protein shell of the virus, scientists can make AAV vectors that have a natural affinity for ovarian cancer cells or even for ovarian cancer stem cells. These modifications include the insertion of targeting peptides or mutations that enhance the vector’s ability to bind to cell markers unique to ovarian tumors. In practical terms, this means:
- Increased Tumor Specificity: Modified AAV vectors may preferentially infect cancer cells, sparing healthy tissue and reducing side effects.
- Enhanced Penetration: With engineered capsids, the vector can better navigate the tangled network of abnormal blood vessels typical of advanced tumors.
- Improved Efficacy: The selective targeting can boost the overall therapeutic impact by ensuring that critical cancer cell subpopulations are reached.
Diving into Gene Supplementation as a Practical Option
Given the limitations of gene silencing and gene restoration strategies—especially the challenge of achieving complete transduction—many researchers are turning to gene supplementation. This method focuses on delivering therapeutic genes that support the body’s own mechanisms to fight the tumor. For example, supplementing with genes that encode anti-angiogenic proteins or cytokines can help to:
- Stop new blood vessel formation that feeds the tumor.
- Boost the patient’s immune system to recognize and destroy cancer cells.
- Create a more hostile environment for tumor growth, even if not every cancer cell is directly affected.
This approach can have a ripple effect on the tumor environment. Even if only some cells are directly transduced, the proteins they produce can impact neighboring tumor cells, making gene supplementation a critical strategy in the overall treatment portfolio.
Addressing the Blood Supply Dilemma in Tumors
One of the subtle parts of ovarian cancer is its erratic blood supply. The aggressive and uncontrolled growth of ovarian tumors leads to the formation of disorganized vessels that are often leaky and nonfunctional. This off-putting phenomenon makes it tough to deliver therapeutic agents uniformly. Strategies to tackle this problem include:
- Anti-Angiogenic Therapies: Using AAV vectors to deliver genes that produce anti-angiogenic factors can help normalize the blood vessels, potentially enhancing drug delivery.
- Combination Treatments: When gene therapy is paired with conventional chemotherapy, the combined effect may lead to improved outcomes, as the chemotherapy can directly kill tumor cells while the gene therapy helps cut off the tumor’s nutrient supply.
These measures not only address the immediate challenge of drug delivery but also play a role in reducing overall tumor growth and spread over time.
Immune Modulation: A Complementary Strategy
Enhancing the body’s immune response is another promising angle for ovarian cancer treatment. AAV-mediated gene therapy can be used to introduce immune-boosting genes, such as those encoding interleukins (e.g., IL-12), which stimulate a more robust attack on cancer cells. The benefits are multifaceted:
- Activation of T Cells: Genes that bolster the production of cytokines can aid in the activation and expansion of T lymphocytes that target tumor antigens.
- Stimulation of Natural Killer (NK) Cells: Enhancing NK cell activity can directly lead to the destruction of cancer cells, particularly those that manage to evade conventional treatments.
- Establishment of Immune Memory: A lasting immune response is key for long-term cancer control, reducing the chance of relapse.
This immune-modulatory approach is a perfect complement to the direct anti-tumor effects of gene supplementation, enabling a dual attack on the disease.
Challenges in Overcoming Therapy Resistance
The challenge of therapy resistance in ovarian cancer is a source of constant worry among oncologists. Due to the tangled issues involving tumor heterogeneity, even the best therapies might fail when a subset of cancer cells escapes treatment. Some of the reasons for resistance include:
- Clonal Expansion: Not every cancer cell receives the therapeutic gene; those that do not may proliferate unchecked over time.
- Altered Tumor Microenvironment: The shifting dynamics within the tumor can lead to new, resistant clones of cells that survive treatment.
- Reactivation of Mutant Genes: In some cases, even when a gene like TP53 is restored to a wild-type state, the presence of mutant proteins may interfere with its function through dominant-negative effects.
These resistance mechanisms serve as a reminder that while AAV-based gene therapy holds promise, it is not a magic bullet. Instead, the future of ovarian cancer treatment likely lies in combination therapies that address both direct tumor cell eradication and the supportive environment that fosters resistance.
Using AAV Vectors Alongside Traditional Therapies
Given the nerve-racking complexity of ovarian cancer treatment, experts suggest that AAV gene therapy might work best when it is not used in isolation. Combining AAV-based gene supplementation or immune modulation with standard treatments such as cytoreductive surgery, platinum-based chemotherapy, and PARP inhibitors may yield the best results. The advantages of a combination approach include:
- Extended Progression-Free Survival: By addressing multiple pathways of tumor growth, combination therapy can lengthen the time patients remain in remission.
- Heightened Therapeutic Impact: Standard drugs can reduce tumor volume, while gene therapy can work on preventing regrowth and metastasis.
- Reduced Side Effects: Using lower doses of chemotherapeutic agents in combination with gene-based treatments may reduce the overall treatment toxicity.
In essence, combination treatments embrace a multifaceted approach that targets both the cancer cells and their supportive microenvironment, leading to more promising long-term outcomes.
Key Considerations for Future Research
While the potential of AAV gene therapy is exciting, several key considerations need attention in future research. Scientists and clinicians must tackle the following aspects to make these therapies more effective and widely applicable:
- Vector Optimization: Continued improvements in AAV capsid engineering are necessary to enhance targeting specificity and transduction efficiency in ovarian cancer cells, especially in the more recalcitrant cancer stem cell populations.
- Administration Routes: More studies are required to compare the benefits of intravenous versus intraperitoneal delivery. Researchers must figure a path through the anatomical challenges to ensure that the vectors reach all parts of the tumor.
- Regulated Gene Expression: Incorporating inducible systems—such as the Tet-On system—can allow doctors to control when and how much therapeutic gene is expressed. This fine-tuning may reduce side effects and provide more precise treatment timing.
- Combination with Immune Therapies: Pairing gene therapy with immune checkpoint inhibitors or CAR-T cell therapy might prove to be a super important strategy, as it attacks the tumor on multiple fronts.
Potential Long-Term Benefits and Patient Impact
Should the obstacles be overcome, the potential benefits of AAV gene therapy for ovarian cancer are significant. The extended expression of therapeutic genes could not only extend progression-free survival but might also contribute to reducing the likelihood of tumor relapse. For patients facing this intimidating disease, the future could hold:
- Longer Lifespans: By effectively managing tumor growth and recurrence, gene therapy can provide patients with more quality years of life.
- Improved Quality of Life: With reduced reliance on repeated cycles of toxic chemotherapy, patients may experience fewer side effects and a better overall well-being.
- More Personalized Treatment: Advances in ovarian cancer organoid models offer the chance to test various gene therapy approaches on a patient’s own tumor cells. This personalization helps fine-tune treatment plans to suit individual cases.
These benefits, however, are intertwined with many complicated pieces of biological research and clinical trial designs. It will require close collaboration among scientists, clinicians, and regulatory bodies to translate these promising findings into tangible patient outcomes.
Addressing the Hidden Complexities of Clinical Translation
Translating lab research into clinical practice is never easy—there are many fine points that need to be worked through. Researchers must consider not only the safety and effectiveness of the AAV vectors themselves but also the practical issues related to large-scale manufacturing, quality control, and patient selection. Some of the key hidden complexities include:
- Manufacturing and Scalability: Producing enough high-quality AAV vectors for widespread clinical use is a challenge. The process must be both cost-effective and reproducible.
- Patient Immune Response: Despite the lower immunogenicity of AAV, many patients may already have pre-existing antibodies that could neutralize the vectors. Overcoming this hurdle might require innovative strategies, such as using capsid variants with reduced recognition by the immune system.
- Patient Selection: Ovarian cancer varies from case to case, and selecting the right candidates for gene therapy will be key. Biomarker studies and personalized screening methods using cancer organoids could help tailor the treatment to individual needs.
Collaborative Efforts and Multidisciplinary Approaches
No single discipline can solve all the puzzles in ovarian cancer gene therapy. Instead, this field thrives on collaboration among oncologists, molecular biologists, immunologists, and bioengineers. Such multidisciplinary approaches are essential for addressing the following:
- Vector Design Improvements: Molecular biologists and bioengineers work hand in hand to refine AAV capsid structures, ensuring the vectors target the tumor effectively and evade host immunity.
- Robust Preclinical Models: Oncologists and researchers utilize ovarian cancer organoid systems to test the efficacy of gene therapies in a manner that reflects patient reality.
- Clinical Trial Planning: Close collaboration between clinicians and regulatory agencies is necessary to design clinical trials that are safe, efficient, and ethically sound.
These collaborative efforts are the key to ironing out the confusing bits and turning experimental therapies into viable clinical options.
Looking Ahead: What the Future Holds for AAV Gene Therapy in Ovarian Cancer
In summary, the use of AAV for ovarian cancer gene therapy represents an emerging and hopeful frontier. The path is filled with many twists and turns—from the early days of vector design to the eventual integration of gene therapy with traditional treatments. Despite the intimidating challenges and the nerve-racking pace at which tumors can adapt, the incremental improvements in our understanding and technology pave the way for a future where gene therapy could become an integral part of ovarian cancer treatment. Some future directions include:
- Refinement of Capsid Engineering: Ongoing research will likely produce AAV variants that are even better at homing in on both ovarian cancer cells and the supportive tumor environment.
- Personalized Medicine Approaches: Patient-derived tumor organoids could serve as testing grounds to determine the optimal gene therapy strategy for each individual.
- Enhanced Combination Regimens: Integrating gene therapy into combined treatment modalities could significantly extend progression-free survival and improve overall patient outcomes.
The possibility of using gene therapy as a “booster shot” following surgery and chemotherapy is particularly exciting. By administering AAV vectors to deliver key anti-tumor proteins right after initial treatment, there is potential to delay or even prevent relapse, making ovarian cancer a more manageable disease in the long run.
Concluding Thoughts
The journey to effective ovarian cancer treatment is full of tricky parts, tangled issues, and nerve-racking challenges. However, by taking a closer look at the potential of AAV gene therapy, we can see a promising path forward—a path that leverages the strengths of gene supplementation, immune modulation, and precision targeting. With continued research, improved vector engineering, and multidisciplinary collaboration, gene therapy may well become a critical tool in our arsenal against ovarian cancer.
While many uncertainties remain, the steady progress in understanding the fine details of ovarian cancer biology and gene delivery systems fuels optimism. For patients facing the daunting reality of ovarian cancer, innovative approaches such as AAV gene therapy offer a glimmer of hope—a hope grounded in science, driven by collaboration, and focused on delivering a lasting impact on survival and quality of life.
As we continue to work through the twists and turns of this exciting field, one thing becomes clear: the future of ovarian cancer treatment is evolving. The sustained efforts to transform experimental gene therapies into practical, life-changing treatments remind us that even the most overwhelming medical challenges can be met with creativity, persistence, and innovative science.
Ultimately, AAV gene therapy may not just be a breakthrough for ovarian cancer—it could be a transformative leap for gene therapy as a whole, setting the stage for a new era of personalized, precise, and long-lasting cancer treatments.
Originally Post From https://www.nature.com/articles/s41417-025-00926-4
Read more about this topic at
AAV for ovarian cancer gene therapy
Adeno-associated virus vector-mediated gene therapy for …