Innovative Immunotherapeutic Perspectives in Blood Cancer Treatment
Recent advancements in cancer research have introduced a promising immunotherapeutic strategy that reprograms the mode of cell death in malignant B cells. This breakthrough approach, which shifts the balance from a silent cell demise to an inflammatory, immunogenic pathway, has the potential to change the way we treat hematological cancers, including certain lymphomas and leukemias. In this editorial, we take a closer look at the study’s methods, challenges, and promising outlook as well as the broader implications for modern medicine.
Reprogramming Cell Death: Shifting from Silent Apoptosis to Immunogenic Necroptosis
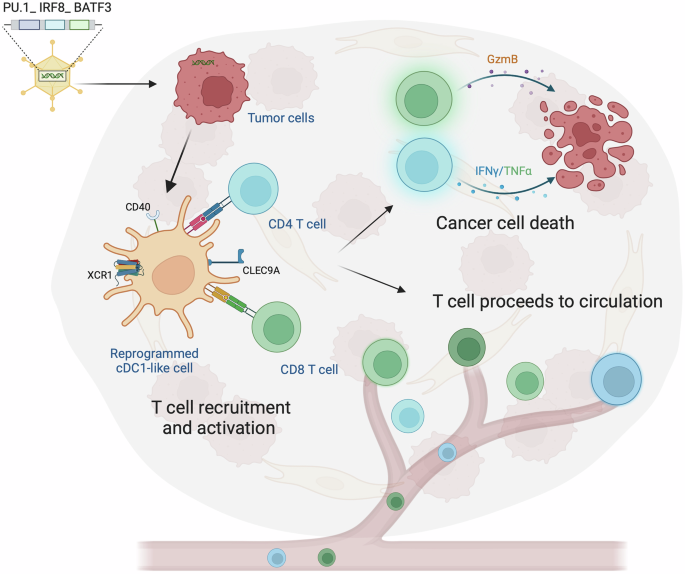
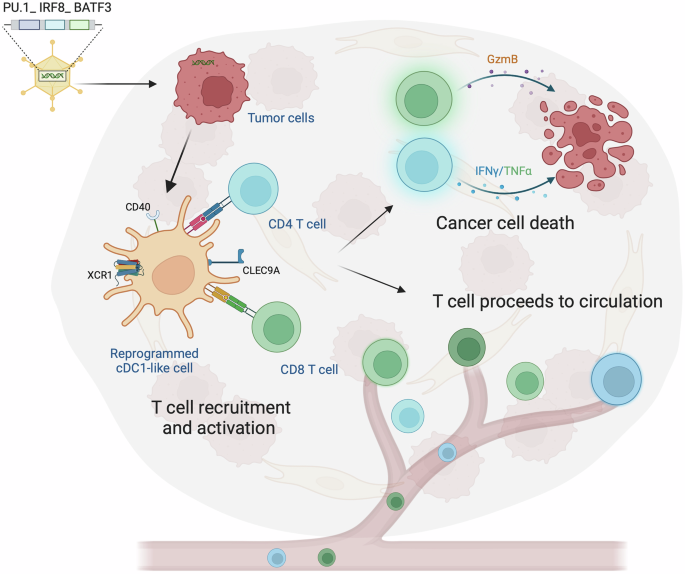
Traditional cancer therapies have long relied on inducing apoptosis—a process of programmed cell death that is generally non-inflammatory and quiet. However, the silent nature of apoptosis often means that malignant cells do not effectively alert the immune system about their presence. The groundbreaking study in question marks a pivot toward necroptosis. Unlike apoptosis, necroptosis releases danger-associated molecular patterns (DAMPs) that encourage a robust immune response. These danger signals help to recruit immune effector cells, essentially turning dying tumor cells into an in situ vaccine.
This approach could be seen as a game-changer in how we approach blood cancers. By ensuring that the cell death process is inflammatory, researchers hope to overcome some of the tangled issues surrounding antigen presentation and immune system redundancy. In simpler terms, malignant cells are not only eliminated but also serve as triggers for ongoing immune surveillance.
Key Benefits of Inducing Immunogenic Cell Death
- Enhanced Immune Activation: Necroptosis provides a boost to the host immune defenses by releasing signals that attract macrophages and T cells.
- In Situ Vaccination Effect: The dying cancer cells help prime the immune system, potentially leading to long-lasting tumor control.
- Overcoming Immune Evasion: By making tumor antigens more visible, this method may reduce relapses and improve survival outcomes.
Addressing the Tricky Parts in B Cell Immunotherapy
One of the most interesting—and at times intimidating—challenges in this research was the molecular barrier found in malignant B cells. Specifically, the absence of a key effector protein, MLKL, which is crucial for executing necroptosis. Without MLKL, the cells were not able to undergo this inflammatory form of cell death, which could have limited the therapy’s effectiveness.
To get around this tricky issue, researchers engineered a triple drug regimen using clinically approved agents. This innovative combination bypassed the missing protein’s requirement, reactivating necroptotic pathways within the cancer cells. The benefit of using already approved drugs is significant; it means that the timeline for transitioning into human clinical trials could be considerably shortened.
Strategies Used to Overcome Molecular Barriers
The team’s approach can be summarized as follows:
- Combination Therapy: The use of three drugs in a novel sequence and dosage was critical to restore the necroptotic pathway.
- Bypassing MLKL Dependence: The regimen successfully navigated around the absence of MLKL, which had been a major deterrent in previous attempts.
- Preclinical Success: The animal models treated with this approach achieved complete leukemia clearance, a promising marker for future clinical efficacy.
Utilizing Advanced Intravital Imaging: A Closer Look at Two-Photon Microscopy
A key part of the study’s success was the application of state-of-the-art intravital two-photon microscopy. This advanced imaging method allowed researchers to watch cellular interactions in real time, directly observing the dynamics between dying tumor cells and immune cells.
Two-photon microscopy offers several advantages, especially when compared to conventional imaging methods. Its ability to provide deep tissue images with minimal photodamage makes it an essential tool not only in this study but also for future research into the small distinctions in tumor microenvironments.
Benefits of Two-Photon Intravital Microscopy
| Feature | Advantage |
|---|---|
| Deep Tissue Imaging | Enables visualization of cell interactions in living tissues, revealing fine shades of immune response dynamics. |
| Real-Time Visualization | Allows researchers to dig into the timing and spatial organization of immune cell recruitment to the tumor site. |
| Minimal Photodamage | Reduces interference with tissue function, ensuring that natural cell behavior is observed. |
Understanding the Benefits for Hematological Malignancies
Blood cancers, such as those affecting B cells, represent some of the trickiest parts of oncology due to their ability to hide from the immune system. Traditional therapies sometimes struggle with issues like relapse and resistance, often due to the cancer cells’ subtle ways of evading immune detection. The new approach, by making the tumor cells themselves more provocative to the immune system, offers a fresh perspective on how to achieve durable remissions.
By reprogramming cell death to activate the immune system, this therapy could address several of the tangled issues that have haunted hematological malignancies over the years. Instead of just killing the cells, the method educates the immune system—a key step in reducing relapse rates and improving long-term outcomes.
How This Strategy Benefits Patients
There are several key points regarding patient benefit:
- Enhanced Tumor Visibility: Making cancer cells more noticeable to the immune system can help in early and effective detection.
- Improved Immune Memory: With the in situ vaccination effect, the immune system may “remember” and more effectively combat recurring cancer cells.
- Potential for Reduced Relapse: By continuously stimulating the immune system, this therapy could lead to fewer cases of tumor regrowth.
Modulating the Tumor Microenvironment
Another promising aspect of this research is the insight it offers into the role of the tumor microenvironment. Historically, our understanding of how the surrounding tissues influence therapy outcomes has been limited by a focus on the cancer cells alone. In contrast, the new approach takes into account the full spectrum of interactions in the tumor locale.
Through the induced necroptosis, local inflammatory cues are enhanced, reshaping the immune landscape in a way that favors anti-tumor activity. Altering the microenvironment not only aids in direct tumor clearance but also sets the stage for sustained immune function, effectively reducing the chance of tumor regrowth.
Key Elements of the Tumor Microenvironment Transformation
- Inflammatory Signal Amplification: Increases production of cytokines and chemokines that call immune cells to the battlefront.
- Immune Cell Recruitment: Enhances the migration of key players such as macrophages and cytotoxic lymphocytes to the tumor site.
- Long-Term Immune Priming: Creates a more sustained and attentive immune presence, functioning as a continuous monitoring system against cancer cells.
Clinical Translation and Moving Toward Human Trials
While these results from preclinical models are promising, a major question remains: how quickly can these findings be translated into effective human therapies? Given that the drugs used in the triple therapy are already approved for clinical use, there is a bright prospect that this approach could move into human trials faster than entirely new compounds.
Transitioning from animal models to patients is never straightforward—the path is filled with tricky parts such as dosing adjustments, side effect profiles, and fine tuning the therapeutic regimen. However, the pioneering nature of this research might serve as a blueprint, streamlining the process and shortening the time it takes for patients to benefit from this innovative method.
Steps Toward Clinical Implementation
Key steps that researchers and clinicians will need to consider include:
- Optimization of Drug Dosages: There is a need to figure a path for appropriate dosing in humans that mirrors the preclinical successes.
- Safety Evaluations: Rigorous testing must be done on the therapeutic cocktail to ensure minimal side effects.
- Patient Selection Criteria: Identifying which patients are most likely to benefit from such a treatment is critical for both early-phase and subsequent trials.
- Long-Term Outcome Measures: Evaluating the immune memory and sustained response after therapy will help determine the real-world potential of the treatment.
Weighing the Benefits Against the Challenges
As with any cutting-edge therapy, there are both exciting prospects and significant, nerve-racking challenges to address. The novel approach of reprogramming cell death comes with the promise of an immuno-oncology revolution, yet it also presents some complicated pieces that need careful consideration.
The advantages of harnessing necroptosis for treating blood cancers include improved antigen presentation, targeting of immune evasion tactics, and the promise of more durable responses. On the flip side, the therapy demands an in-depth understanding of the small distinctions in immune cell behavior along with painstaking adjustments in drug combination therapy.
Evaluating the Pros and Cons
| Aspect | Pros | Cons |
|---|---|---|
| Mechanism of Action |
|
|
| Clinical Applicability |
|
|
| Immune Engagement |
|
|
Implications for Future Cancer Therapies
The success of this study could pave the way for a new era in cancer treatment, one that integrates molecular insights with the body’s natural defenses. As we continue to get into the role of programmed cell death in cancer therapy, we might witness a shift in focus from simply killing cancer cells to empowering the body to fight the disease more effectively.
In particular, this shift toward harnessing necroptosis is a reminder that the immune system holds tremendous power when properly called upon. Future research might well explore other forms of cell death and the subtle parts of immune activation, deepening our understanding and expanding our arsenal against various forms of cancer.
Future Research Directions
Researchers may consider exploring:
- Combination with Other Immunotherapies: How does this approach synergize with checkpoint inhibitors or CAR-T cell therapies?
- Expanding the Technique: Can the principles behind necroptosis induction be applied to solid tumors or other malignancies?
- Long-Term Immune Memory: What are the fine points of immune memory formation post-treatment, and how can they be optimized?
- Microenvironment Modulation: Further investigations into the role of the tumor microenvironment can help identify additional ways to encourage sustained anti-tumor activity.
Integrating Alternative Medicine and Nutritional Insights
While the recent breakthrough is firmly rooted in molecular biology and immunotherapy, it opens the door to a wider conversation about the role of complementary approaches in cancer care. Alternative medicine, nutrition, and lifestyle factors have all shown potential in modulating immune responses and managing inflammation. Although these approaches are not a substitute for the high-precision treatments discussed, they can form part of an overall strategy to enhance health and well-being.
For instance, specific dietary choices may influence immune function and could bolster the patient’s response to immunotherapy. Similarly, integrative practices like stress reduction and targeted exercise might improve overall outcomes by optimizing the body’s defense systems. These areas remain full of problems and tangled issues that require more research, but they represent essential components of a holistic approach to cancer care.
How Complementary Approaches Can Support Immunotherapy
Some potential benefits include:
- Dietary Interventions: Consuming nutrients that reduce inflammation can help set a more favorable baseline for immune activation.
- Herbal Supplements: Certain herbs have immunomodulatory properties that may work synergistically with targeted therapies.
- Lifestyle Modifications: Stress management and regular physical activity can support the body’s natural immune responses, aiding in long-term treatment success.
Concluding Thoughts: Charting a New Course in Cancer Immunotherapy
In summary, the reprogramming of malignant B cell death from a silent process to an inflammatory, immunogenic event is a super important breakthrough. This innovative approach not only harnesses the body’s own defenses but also turns the battle against cancer into a more dynamic engagement, in which the immune system is actively recruited and trained to fight off relapse.
While there remain several nerve-racking and complicated pieces to sort out, the potential benefits for patients with blood cancers are immense. With the support of advanced imaging technologies, innovative drug regimens, and an improved understanding of the tumor microenvironment, this strategy could redefine what is possible in immuno-oncology.
Looking ahead, it is crucial to maintain a balanced perspective that acknowledges both the exciting possibilities and the challenging bits. As researchers continue to poke around and refine these techniques, the hope is that this new treatment paradigm will soon move from preclinical models to practical, life-saving therapies available to cancer patients across the globe.
Key Takeaways
- Reprogrammed Cell Death: Shifting from silent apoptosis to necroptosis transforms tumor cells into stimulators of the immune response.
- Overcoming Molecular Barriers: Innovative three-drug regimens have effectively bypassed key deficiencies, such as the absence of MLKL.
- Advanced Imaging: Intravital two-photon microscopy has provided a real-time window into the fine details of immune cell interactions within the tumor microenvironment.
- Clinical Prospects: With many of the drugs already approved for other uses, this approach holds promise for rapid clinical translation.
- Holistic Considerations: Integrating nutritional and alternative medicine insights may further support and enhance patient outcomes in cancer therapy.
Final Reflections
The study we have explored is a beacon of hope within the evolving field of immunotherapy. It reminds us that the journey toward effective cancer treatments is not a straight line, but rather a path filled with twists and turns, tangled issues, and plenty of opportunities to innovate. By reprogramming cell death and harnessing the inherent power of the immune system, researchers are opening up new avenues that could ultimately lead to more durable, long-term cancer control.
As we look to the future, it is important for the scientific community, clinicians, and even lifestyle and alternative medicine practitioners to keep an open mind. The integration of modern molecular techniques with broader health strategies may well be the key to conquering some of the most intimidating challenges in cancer therapy.
In the end, sustained progress in this field will depend on continued collaborative research, robust clinical trials, and an unwavering commitment to addressing both the noticeable and subtle parts of the disease process. The promise of transforming malignant B cells into active allies against cancer represents not just a technological advance, but a paradigm shift—a new way of thinking about how we can empower the body’s own defenses to fight one of humanity’s most persistent foes.
With each step forward, we are reminded that while the road ahead is full of problems and nerve-racking uncertainties, it is also paved with hope, opportunity, and the vibrant potential to change lives for the better. Let us keep pushing the boundaries of what is possible in cancer care.
Originally Post From https://bioengineer.org/revolutionizing-blood-cancer-treatment-reprogramming-cancer-cell-death-to-activate-the-immune-system/
Read more about this topic at
Blood cancer: Scientists reprogram cancer cell death to …
Reprogramming of Cell Death Pathways by Bacterial …