Revolutionizing Biomedical Research: The Promise of Organoid Engineering
In recent years, the field of organoid engineering has emerged as a groundbreaking frontier in biomedical research. These miniaturized, three-dimensional cellular constructs offer a unique window into organ development, disease progression, and personalized drug responses. With organoid models becoming increasingly realistic and adaptable, research laboratories and clinicians alike are now presented with the opportunity to study living tissue in ways that traditional two-dimensional cultures or animal models have struggled to match.
As the science behind these in vitro systems matures, a number of advanced and sophisticated techniques have been refined to overcome the tricky parts and tangled issues that once limited our understanding of human biology. This opinion editorial dives into the current state of organoid engineering, underscoring recent innovations, emerging challenges, and future prospects. We will examine critical methods such as air-liquid interface culture systems, bioreactor architectures, vascularization approaches, and the integration of gene editing and bioprinting technologies.
Breaking Ground in Three-Dimensional Tissue Models
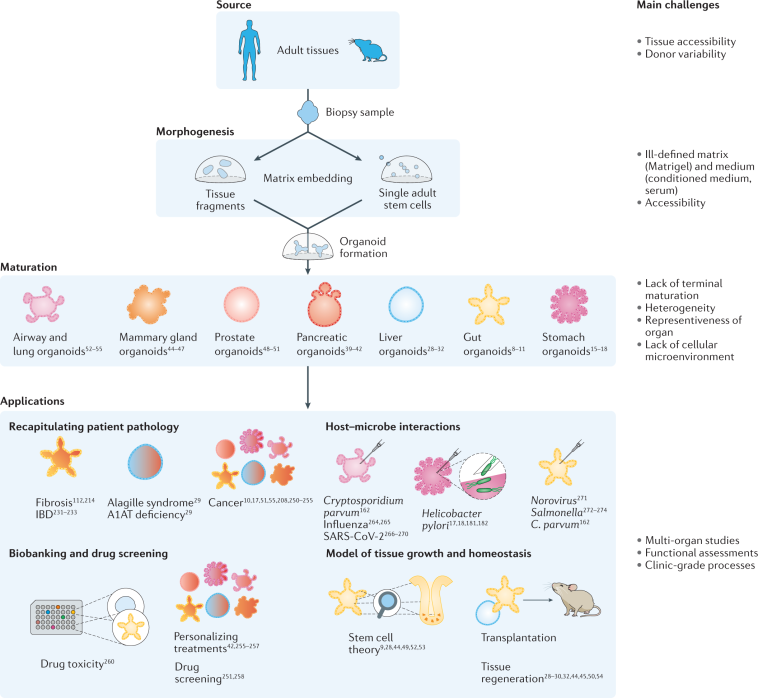
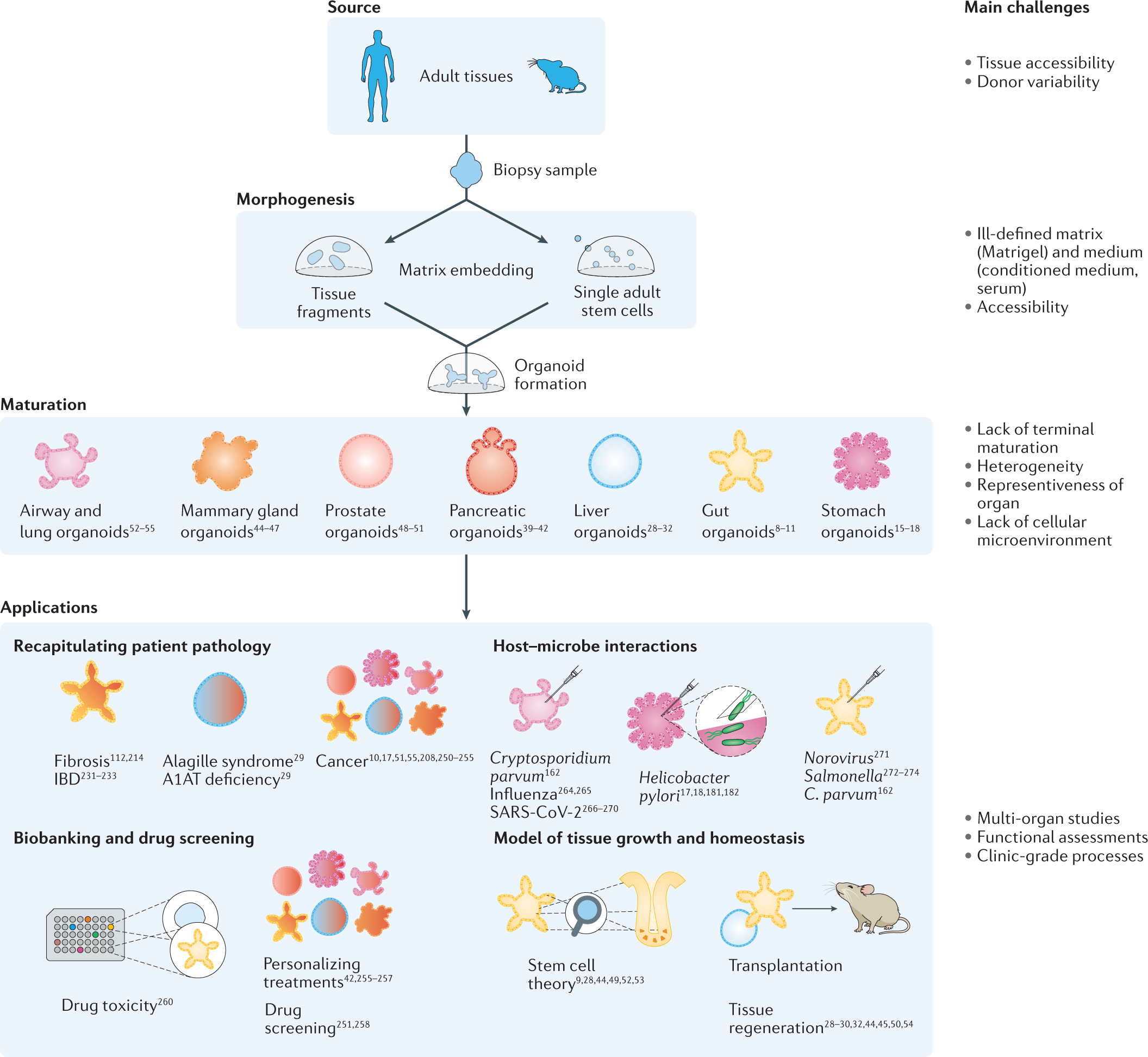
The development of organoids represents a transformational leap from traditional cell cultures by recapitulating the fine structures and functions of human organs. Researchers have long faced intimidating obstacles when trying to reproduce the natural complexity of tissues. With organoids, however, the field has seen exciting progress toward creating models that reflect the subtle details of real organ systems—from the kidneys and liver to the lungs and brain.
Air-Liquid Interface Systems: Mimicking Natural Microenvironments
One of the most effective methodologies in organoid engineering is the air-liquid interface (ALI) culture system. This technique involves exposing one surface of a cell layer to air while keeping the opposite side in contact with a nutrient-rich medium. Such an approach has particularly proven useful when constructing organoids that mimic hollow organs like the lungs and intestines.
Some of the key benefits of the ALI method include:
- Preservation of epithelial integrity in fragile tissues
- Effective co-culturing with immune cell populations
- The ability to reproduce small but critical little twists in the architecture of organs
By maintaining these conditions, researchers can better capture the complex interplay between tissue surfaces and external environments. This helps in exploring how immune cells interact with tumors in lung tissue, for instance, and supports a more authentic recreation of cancer biology than traditional models.
Bioreactor Cultures: Scaling Up with Controlled Environments
Another innovative approach in organoid engineering is the utilization of bioreactor cultures. These systems enable controlled agitation and a steady supply of nutrients, thereby boosting the scalability and complexity of tissue models. In many cases, bioreactor strategies help overcome nerve-racking production challenges and improve tissue differentiation on a larger scale.
Among the benefits of bioreactor techniques are:
- Enhanced growth and maturation of organoids, especially those modeling cerebral structures
- A robust platform for high-throughput screening and drug-testing applications
- Promotion of the formation of vascular-like networks
This method becomes indispensable when tackling larger, more complicated pieces of tissues. In the same way that a well-tuned orchestra needs every instrument to play in harmony, bioreactors help synchronize cellular growth so that the resulting organoids reflect in vivo physiology more accurately.
Vascularization: Breathing Life into Miniature Organs
One of the most promising frontiers in organoid research is the achievement of vascularization. Traditional three-dimensional cultures often fell short in mimicking blood flow, leading to nutrient deprivation and limited tissue survival. Modern vascularization techniques are overcoming these challenges by integrating microvessel networks within organoids.
Vascularized organoids present several key advantages:
- Improvement of nutrient and oxygen diffusion throughout the tissue
- Enhanced maturation and survival rates for delicate cell populations
- The ability to simulate neurovascular interactions that are essential for characterizing disease states
These advances are not without their tricky parts. Successfully integrating vascular channels demands that researchers figure a path through plentiful technical hurdles, including nutrient gradients and physical constraints of three-dimensional growth. Nevertheless, when achieved, these improvements bring the tissue model one step closer to accurately replicating living organs.
Techniques for Improved Vascular Integration
To achieve effective vascularization, scientists are employing several techniques:
- Use of extracellular scaffolds that encourage vessel ingrowth
- Co-culturing with endothelial cells to form capillary networks
- Adoption of microfluidic devices to simulate perfusion-like conditions
These methods, while sometimes intimidating due to their labyrinthine details, also open pathways for enhanced translational applications—ranging from disease modeling to tissue regeneration.
Precision Medicine Meets Organoid Technology
Organoid engineering is poised to revolutionize clinical decision-making by bolstering the era of precision and personalized medicine. Patient-derived organoid biobanks are already reshaping how researchers assess drug efficacy and safety across diverse genetic backgrounds—and importantly, they do so with a high degree of accuracy compared to classic animal models.
Application in Drug Discovery and Toxicity Testing
Traditional animal testing has long been a standard in evaluating new therapeutic compounds. However, species-specific differences often hinder the translation of preclinical findings to human outcomes. Organoid models offer a much-needed alternative, providing human-based tissue platforms that can better predict drug metabolism and adverse reactions.
In drug discovery and toxicity assessment, the benefits of using organoids become apparent:
- Realistic representation of human-specific physiology and cellular architecture
- Enhanced reliability when screening candidate drugs for safety and potency
- A reduction in failure rates during clinical trial phases
Moreover, these three-dimensional tissue structures facilitate a more nuanced evaluation of compounds. This, in turn, can streamline regulatory approval processes by minimizing the confusing bits of drug development, such as unexpected toxicity or inefficacy.
Blending Modern Science with Traditional Medicine
One of the lesser-discussed yet equally exciting applications of organoid technology lies in its capability to modernize Traditional Chinese Medicine (TCM). By employing simplified but cutting-edge in vitro models, researchers can now poke around the effectiveness and mechanisms of herbal compounds, transforming ancient practices into scientifically validated treatments.
Modernizing TCM Through Organoid Research
Traditional Chinese Medicine has relied on holistic approaches for centuries, yet the scientific community has often found it difficult to dissect the small distinctions and subtle parts inherent in these treatments. Organoid models offer a controlled setup that allows for:
- Active component screening in a physiologically relevant environment
- Mechanistic elucidation of multi-target effects within a single organ model
- Toxicity profiling and validation of herbal mixtures with modern scientific rigor
This integration paves the way for an evidence-based approach to TCM. The ability to translate traditional practices into quantifiable parameters not only bolsters clinical confidence but also lays the foundation for complementary medicine strategies that blend the best of modern and traditional paradigms.
Future Prospects: Gene Editing and Bioprinting Technologies
While current advances in organoid engineering are impressive, the field is far from reaching its full potential. Emerging technologies such as CRISPR-Cas9 gene editing and three-dimensional bioprinting are poised to take these systems to the next level, thereby smoothing the path for even more sophisticated disease models.
Gene Editing: Customizing Organoid Models
CRISPR-Cas9 has revolutionized our capability to make precise genetic modifications. In the context of organoid engineering, this tool allows scientists to build models with patient-specific mutations, affording researchers the chance to identify gene-disease associations in a controlled setting.
Key applications of gene editing within organoid research include:
- Creating bespoke disease models that mirror specific genetic mutations
- Poking around the roles of individual genes in tissue development and pathology
- Facilitating functional assays to explore the effects of genetic alterations
By embracing these techniques, researchers can cut through the confusing bits of genetic variability to deliver models that are both clinically relevant and scalable for drug screening.
Three-Dimensional Bioprinting: Crafting Tissues with Precision
The use of 3D bioprinting has opened up exciting avenues for fabricating organoids with defined spatial architectures. This technology allows for the automated, layer-by-layer assembly of cells, scaffolds, and extracellular matrices, leading to highly reproducible and structurally intricate tissue models.
Benefits of 3D bioprinting in organoid engineering include:
- The ability to control the arrangement and orientation of cells within an organoid
- Improved reproducibility and scalability—addressing one of the most intimidating challenges of organoid production
- Potential applications in regenerative medicine where tailored tissue replacements could be fabricated on-demand
While the use of bioprinting is still in its early stages, its promise in creating advanced, patient-specific tissues is both super important and highly anticipated for future therapeutic applications.
Tackling the Tricky Parts: Challenges and Considerations
No discussion of organoid engineering would be complete without addressing the many tricky parts and tangled issues that still lie ahead. As promising as these systems have become, several challenges continue to limit their widespread clinical adoption.
Standardization and Reproducibility Across Laboratories
One of the main challenges in the field is ensuring that organoid models can be consistently reproduced across different research centers. Variations in cell sourcing, culture conditions, and even subtle differences in technique can lead to discrepancies in the final tissue model. For example, what works beautifully in one laboratory might prove to be less effective in another, leading to a landscape that is still somewhat on edge.
Table 1 below summarizes some common issues and possibilities for resolution:
| Challenge | Possible Solution |
|---|---|
| Variability in Stem Cell Sources | Use standardized cell lines and share biobank resources |
| Differences in Culture Conditions | Develop and adhere to universally accepted protocols |
| Reproducibility Issues Between Labs | Promote collaborative studies and cross-validation of techniques |
Efforts to standardize these methods remain a key priority as the field moves toward broader clinical application. By addressing the confusing bits and nerve-racking production challenges, the community can pave the way for more reliable, reproducible models.
Cost and Resource Allocation
Another daunting aspect is the economic side of organoid research. The methods used to cultivate these three-dimensional models—especially when incorporating advanced features like vascularization or bioreactor cultures—often require significant capital investment. As a result, making these techniques more accessible and affordable for routine clinical and research settings is a super important goal.
Strategies to manage these costs include:
- Scaling up production through automation and bioprinting
- Encouraging open-source collaboration to share protocols and resources
- Investing in technology that reduces waste and shortens culture times
Addressing these economic hurdles is critical, as streamlined production processes could eventually lead to a decrease in overall costs, making personalized medicine applications feasible on a broader scale.
Integrating Computational Analytics and Artificial Intelligence
Alongside physical innovations, the integration of computational tools and artificial intelligence (AI) holds great promise for the future of organoid engineering. Complex organoid datasets—riddled with subtle parts and nuanced differences—are becoming increasingly manageable through the use of advanced data analytics.
AI-Driven Insights into Development and Pathology
Artificial intelligence can process and interpret vast amounts of data generated by organoid experiments. These AI platforms help in:
- Identifying patterns and correlations that are not immediately obvious to human observers
- Predicting how variations in culture conditions might affect tissue development
- Optimizing experimental designs by highlighting slight differences and fine shades in response profiles
This AI-enhanced approach not only aids in the day-to-day management of these models, but it also accelerates the pace of discovery in both fundamental research and therapeutic applications.
Using Single-Cell Sequencing to Decode Hidden Complexities
Another emerging tool is single-cell RNA sequencing, which allows researchers to get into the nitty-gritty details of cellular processes at an unprecedented resolution. Combined with computational analysis, this technique can:
- Reveal the subtle parts of gene expression changes over time
- Help in dissecting the fine points of cell lineage and differentiation
- Improve our understanding of how individual cells contribute to overall tissue function
By leveraging these technologies, scientists can manage their way through the dizzying matrix of gene-expression data to create more accurate models of both healthy and diseased states.
Charting a Path Forward: Collaborative Endeavors and Future Prospects
Despite the intimidating challenges and complicated pieces that still exist in organoid engineering, the potential rewards are immense. The promising results so far have spurred an increasing amount of collaborative effort across research institutions, biotechnology companies, and clinical centers.
Interdisciplinary Collaborations: A Must-Have for Future Progress
The complexity of organoid engineering—marked by the twists and turns of molecular biology, engineering, and computational analytics—necessitates close teamwork among experts from various fields. Successful initiatives often incorporate insights from:
- Biomedical engineers who optimize bioreactor conditions and implement bioprinting technologies
- Molecular biologists who refine gene editing techniques to make patient-specific organoids
- Data scientists who streamline the analysis of complex datasets
- Clinicians who bridge the gap between laboratory findings and patient care
Together, these interdisciplinary teams can sort out the tangled issues of organoid research and make steady progress toward clinically relevant breakthroughs.
The Road Ahead: Making Organoid Engineering Mainstream
Looking to the future, several factors will be key in determining whether organoid engineering can transition from a high-potential research tool to a staple of personalized medicine:
- Developing universally agreed-upon protocols for production and analysis
- Addressing the economic challenges through streamlined, automated processes
- Ensuring reproducibility across laboratories by developing robust quality control measures
- Integrating emerging technologies such as AI and single-cell analytics to further refine models
Once these critical steps are taken, organoids have the potential to transform our approach to drug discovery, toxicity testing, and regenerative medicine in ways that more accurately reflect human biology and disease mechanisms.
Conclusion: Embracing the Future with Cautious Optimism
As our understanding of organoid engineering deepens, it is clear that these three-dimensional models offer a powerful platform for addressing some of the trickiest parts of biomedical research. By working through the tangled issues—whether it be in scaling up production, ensuring reproducibility, or integrating sophisticated computational tools—researchers are steadily steering through the path toward personalized, predictive, and integrative medicine.
While there remain numerous nerve-racking challenges and overwhelming production costs to be tackled, the transformative impact of this technology is already evident. Organoids not only promise to bridge the gap between preclinical research and clinical application but also pave the way for a future where medical treatments are tailored to the individual nuances of each patient’s biology.
The journey is far from over. Every step taken—whether it involves refining air-liquid interface techniques, optimizing bioreactor conditions, or embracing revolutionary approaches like gene editing and bioprinting—moves us closer to a paradigm where the lab bench and the bedside are interconnected like never before.
In this exciting era, collaboration, innovation, and the collective drive to resolve the confusing bits and small distinctions in tissue engineering are the keys to success. As researchers continue to make headway in unravelling the fine points of organoid development and function, it is essential to maintain a measured yet optimistic perspective on the future of personalized medicine.
Through the combined efforts of scientists from multiple disciplines and by leveraging cutting-edge technology, the promise of organoid engineering will soon transition from an experimental marvel to an indispensable tool in modern clinical practice. The emerging synergy between modern biomedicine and traditional healing methods further solidifies the role of organoids in shaping tomorrow’s healthcare landscape.
In summary, organoid engineering is a beacon of hope that offers not only a deeper understanding of human biology but also ushers in a transformative era for drug development, disease modeling, and regenerative medicine. As we continue to dig into these scientific frontiers, it is clear that the journey – with all its twists and turns – is one worth pursuing, for it holds the key to a future of more accurate, personalized, and effective medical care.
Originally Post From https://bioengineer.org/breakthroughs-in-organoid-engineering-advanced-construction-techniques-model-innovations-and-pathways-to-clinical-application/
Read more about this topic at
Revolutionizing Disease Modeling: The Emergence …
Recent Advances in Applications of Nanoparticles and …