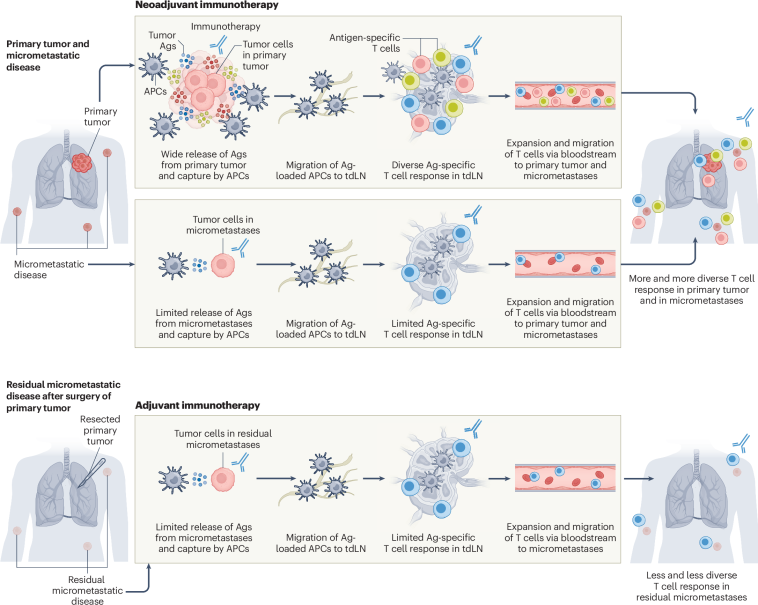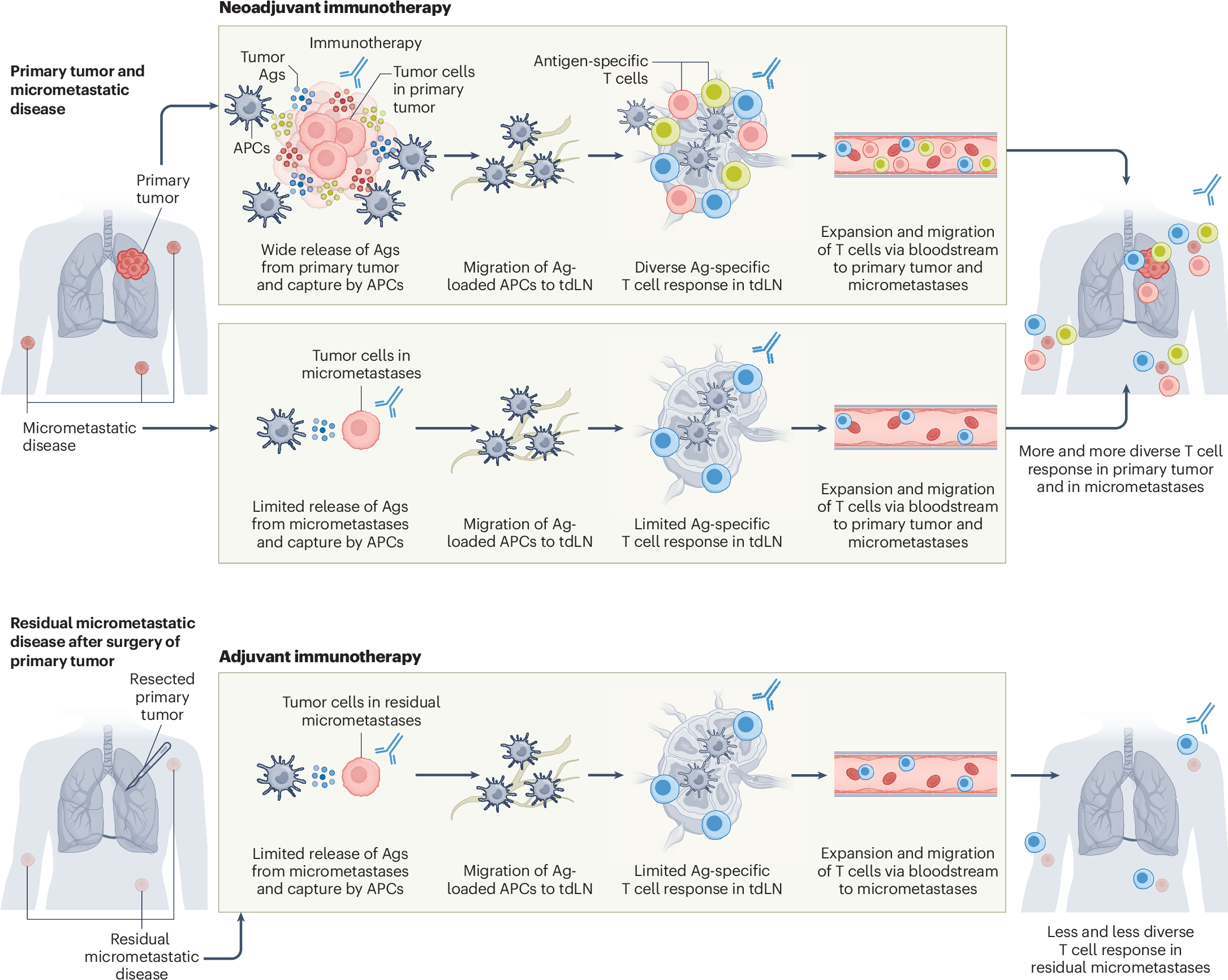
Innovative Neoadjuvant Approaches in Bladder Cancer: A Closer Look at TAR-200 Plus Anti–PD-1 Therapy
At the recent European Society of Medical Oncology (ESMO) Congress 2025, a significant discussion emerged around an emerging intravesical gemcitabine delivery system, TAR-200, used in combination with the anti–PD-1 antibody sacituzumab. This combination has demonstrated promising neoadjuvant activity in patients with muscle-invasive bladder cancer who are ineligible for cisplatin-based therapies. Today, we take a closer look at the SunRISe-4 trial results, discussing the key outcomes, the trial’s safety profile, and potential implications on shifting treatment paradigms in bladder cancer management.
Breaking Down the SunRISe-4 Trial: Key Findings and Their Implications
The SunRISe-4 trial, first previewed at ESMO 2024 and later detailed in The Lancet Oncology, has generated considerable enthusiasm within the oncology community. The trial enrolled patients with clinical stage T2–T4a, N0, M0 muscle-invasive urothelial carcinoma who either were not eligible for or declined cisplatin-based chemotherapy. Participants were randomized into two groups: one being treated with four cycles of TAR-200 plus sacituzumab and the other receiving sacituzumab monotherapy.
Highlights from the study include:
- Pathologic Complete Response (pCR): The combination therapy achieved a pCR rate of 38%, as opposed to 24% in the monotherapy arm.
- Pathologic Downstaging: More than 50% of patients on the combination regimen experienced downstaging, compared to 44% with only sacituzumab.
- Relapse-Free Survival (RFS): One-year RFS was 77% with the combination treatment, marking a 10% absolute improvement over the 66% RFS rate observed with sacituzumab alone.
These findings spotlight the potential for intravesical therapy to provide an additive benefit when integrated into the pre-surgery, neoadjuvant setting. The nuances of these results are making waves in patient care strategies, particularly in light of the ever-present challenges associated with cisplatin-based regimens.
Tackling the Tricky Parts of Cisplatin-Ineligible Bladder Cancer Patients
Patients who are not eligible for cisplatin-based chemotherapy face a host of tricky parts when it comes to their treatment options. Often, these individuals are left with limited alternatives that might not offer the same therapeutic benefit. For many patients, the inability to tolerate cisplatin can be both intimidating and a source of considerable anxiety. The recent trial results suggest that TAR-200 plus an anti–PD-1 therapy could become a key alternative, offering an approach that steers patients toward better outcomes.
For clinicians and patients alike, this development is a welcome option, especially when considering the following challenges:
- Comorbidities: Many cisplatin-ineligible patients have other underlying health concerns that make traditional chemotherapy a nerve-racking prospect.
- Side Effect Profiles: Cisplatin is notorious for causing severe toxicity, particularly renal impairment and peripheral neuropathy, making alternative regimens super important.
- Treatment Efficacy: Finding a balance between treatment efficacy and tolerance is critical for this patient population.
By introducing a novel intravesical approach that targets the tumor locally with gemcitabine and enhances the immune response via PD-1 blockade, clinicians can potentially offer a less overwhelming yet effective treatment pathway for these patients.
Understanding the Hidden Complexities of Neoadjuvant Therapy Integration
While the trial data look promising, integrating new regimens into established treatment protocols always comes with its own set of complicated pieces. There are several subtle details and little twists to consider when thinking about employing TAR-200 plus sacituzumab in a neoadjuvant setting. Here are some of the most critical factors:
- Patient Selection: Identifying which patients will benefit most from the combination therapy requires careful review of biomarkers, performance status, and overall health.
- Timing of Administration: The scheduling of the four treatment cycles prior to radical cystectomy must be harmonized with surgical readiness and patient recovery times.
- Monitoring Safety: Although the study reported no new or unexpected signals, managing local adverse events like urinary tract symptoms remains an essential part of patient care.
- Long-Term Outcomes: While the one-year relapse-free survival data are encouraging, longer follow-up is necessary to evaluate sustained benefits and overall survival impacts.
These fine points, combined with the overall therapeutic optimism, underscore the importance of a tailored approach to using intravesical therapies in the context of modern oncology care.
The Role of Immunotherapy in Modern Bladder Cancer Care
The integration of immunotherapy into the neoadjuvant treatment landscape is a shift that has been eagerly watched by clinicians. Given the immune system’s potential to target cancer cells, there is a genuine hope that immunotherapy could not only shrink tumors prior to surgery but also prevent recurrence.
When we examine the combination of TAR-200 with sacituzumab, several key considerations arise:
- Immune Activation: The anti–PD-1 antibody works by lifting the brakes off the body’s immune defenses, enabling a more robust attack on tumor cells.
- Synergistic Effects: The localized delivery of gemcitabine complements the systemic immune modulation provided by sacituzumab, potentially leading to a more comprehensive cancer cell kill.
- Quality-of-Life Improvements: Given that adverse events were mostly local and well-managed, patients may experience fewer of the overwhelming side effects typically associated with broad-spectrum chemotherapy.
This dual approach signifies a key advancement in cancer treatment where targeting both the tumor directly and modulating the immune response appears to be super important. It also opens the door to further research exploring other combinations that incorporate local drug delivery systems with immunomodulators.
Comparisons with Cisplatin-Based Regimens: A Shift in the Treatment Landscape
Historically, cisplatin has been the cornerstone of neoadjuvant therapy in muscle-invasive bladder cancer. However, its challenging side effect profile and the substantial proportion of patients unable to tolerate it necessitate the exploration of alternatives. The recent SunRISe-4 findings offer a way to get around these limitations.
Let’s take a look at a simplified table comparing cisplatin-based chemotherapy with the new TAR-200 plus sacituzumab combination:
| Criterion | Cisplatin-Based Chemotherapy | TAR-200 Plus Sacituzumab |
|---|---|---|
| Patient Eligibility | Limited by renal function and comorbidities | Available for cisplatin-ineligible patients |
| Pathologic Complete Response | Historical rates vary, often lower | 38% pCR rate in SunRISe-4 trial |
| Relapse-Free Survival | Generally lower, with higher relapse rates | 1-year RFS at 77% versus 66% for monotherapy |
| Side Effect Profile | High risk of systemic toxicity | Mostly local adverse events, better managed |
This comparison highlights the potential for a less intimidating treatment alternative for patients who have been left with few options. It also stresses that, while cisplatin remains a very important option for eligible patients, having additional strategies available can make a significant difference in how treatment plans are developed.
Addressing the Tricky Parts of Safety and Side Effects
Not every new treatment comes without its own set of tangled issues, and safety is always a paramount concern when evaluating any therapy. In the SunRISe-4 trial, the safety data for the combination of TAR-200 with sacituzumab were reassuring. The study noted that, aside from local adverse events like urinary tract symptoms, few severe complications were encountered.
Some key points regarding safety include:
- Local Versus Systemic Effects: The localized administration of gemcitabine minimizes systemic absorption, reducing the risk of widespread toxicity.
- Manageability of Adverse Events: Most of the grade 3 to 4 adverse events were local and manageable by clinicians, which is critical for patient comfort and treatment adherence.
- Monitoring and Follow-Up: Ongoing monitoring is crucial to ensure that any emerging safety signals are identified and addressed promptly.
These factors suggest that TAR-200 combined with sacituzumab might offer a safer alternative, particularly when the benefits of conventional cisplatin therapy are off-putting or simply out of reach for many patients.
Integrating Novel Therapies into Clinical Practice: Tips for Practitioners
For healthcare providers, the introduction of new treatment modalities often involves sorting out several practical considerations. When adopting a regimen like TAR-200 plus sacituzumab, clinicians may face some confusing bits related to treatment logistics, monitoring protocols, and patient communication. Here are some super important tips for integrating this innovative therapy into clinical practice:
- Thorough Patient Evaluation: Before initiating therapy, ensure that patients undergo comprehensive assessments to confirm their ineligibility for cisplatin and evaluate overall health status.
- Clear Scheduling: Set up a treatment schedule that aligns with surgical planning and postoperative care, making sure that patients understand the timeline and what to expect.
- Effective Communication: Discuss the benefits and potential local side effects in clear, everyday language that patients can easily understand, helping them get around any worries they might have.
- Collaborative Care: Engage multidisciplinary teams—oncologists, urologists, and support staff—to provide a seamless treatment experience, ensuring that every twist and turn is managed effectively.
- Regular Monitoring: Implement robust follow-up procedures to quickly address any adverse events and adjust treatment plans as necessary.
These actionable strategies are part of a broader effort to ensure that, as new combinations and treatment paradigms emerge, healthcare providers are well equipped to deliver safe, effective, and compassionate care.
Exploring the Fine Points of Intravesical Gemcitabine Delivery Systems
TAR-200 represents an innovative step forward in the delivery of chemotherapeutic agents directly to the tumor site. Intravesical therapy has always offered the appeal of reduced systemic toxicity, and TAR-200’s design for the sustained release of gemcitabine is particularly clever.
When we take a closer look at the fine points of such delivery systems, several features stand out:
- Controlled Release: The technology behind TAR-200 ensures a controlled release of gemcitabine, maintaining effective drug levels in the bladder over a prolonged period.
- Localized Impact: Direct application to the tumor site minimizes widespread exposure, which reduces off-target effects and significantly improves tolerability.
- Potential for Combination: When paired with an immunotherapy like sacituzumab, the localized chemotherapy can be enhanced by systemic immune activation, offering a multi-faceted approach against cancer cells.
- Patient Convenience: This method can lessen the need for frequent hospital visits and may improve overall quality of life by reducing the nerve-racking side effects commonly associated with intravenous chemotherapy.
These attributes not only improve patient outcomes but may also serve as a blueprint for future innovations in drug delivery across various solid tumors.
Innovative Immunotherapy Combinations: Opportunities and Challenges
Emerging combination therapies that integrate localized drug delivery with systemic immunomodulation are on the cutting edge of oncology. However, they also come with a set of confusing bits that require careful consideration from both researchers and clinicians.
Some of the key opportunities and challenges include:
- Enhanced Efficacy: Combining therapies can create synergistic effects that lead to improved tumor responses.
- Dose Optimization: Figuring out the right dosing regimen is essential to avoid overloading the patient with multiple side effects while still achieving a therapeutic benefit.
- Cost and Accessibility: As with all novel therapies, ensuring that these advanced treatments remain accessible to a broad patient population is a critical concern.
- Clinician Training: There is a need for continuous education so that healthcare professionals can stay up to date with the latest strategies and subtle details regarding dose adjustments and monitoring.
Addressing these points will be pivotal as the oncology community makes its way through the evolving landscape of personalized cancer care. The promise of these combination approaches could reshape treatment protocols for bladder cancer and beyond.
Long-Term Implications for Patient Outcomes and Quality of Life
Beyond the immediate efficacy markers, such as the pCR rate and one-year relapse-free survival, the long-term implications of introducing therapies like TAR-200 plus sacituzumab are of critical interest. From an opinion standpoint, the potential to improve quality of life stands out as a key benefit.
Areas where these improvements may be most evident include:
- Reduced Systemic Toxicity: With fewer severe systemic side effects, patients may retain better overall health, allowing them to participate in daily activities with fewer interruptions.
- Enhanced Recovery: More patients may be able to proceed to radical cystectomy with less preoperative compromise, potentially reducing the risk of postoperative complications.
- Improved Psychological Outlook: Knowing that there is an effective alternative to the intimidating regimen of cisplatin-based chemotherapy can alleviate fears and contribute to a more positive mindset during treatment.
- Potential for Extended Survival: A 10% improvement in one-year relapse-free survival is not just a statistic—it reflects a significant stride in ensuring patients enjoy longer, healthier lives post-treatment.
These factors collectively underscore the importance of continued research and clinical investment in combination therapies, as the benefits can extend well beyond tumor shrinkage to overall patient well-being.
Changing the Conversation: The Role of the Healthcare Community
The introduction of novel therapies like TAR-200 plus sacituzumab is reshaping conversations across the healthcare spectrum. From oncologists to pharmacists and nursing staff, every team member plays a part in the successful implementation of these innovative regimens. Here are some ways the healthcare community can work together to make the most of this opportunity:
- Interdisciplinary Collaboration: Bringing together specialists from oncology, urology, pharmacy, and supportive care can help smooth out the tangled issues related to treatment planning and patient management.
- Patient Education Initiatives: It is essential to provide patients with clear, digestible information about their treatment options and what they can expect throughout the process.
- Ongoing Research and Data Sharing: Robust data collection and sharing among institutions will help refine dosing strategies, improve safety profiles, and ensure the long-term success of these therapies.
- Advocacy for Access: Healthcare professionals can also advocate for insurance coverage and reimbursement policies that ease the financial burden on patients, making advanced care accessible to more individuals.
By working together and sharing insights, the healthcare community can effectively tackle the complicated pieces associated with integrating new therapies into everyday practice.
Looking Ahead: Opportunities for Future Innovations in Oncology
While the current results from the SunRISe-4 trial are promising, they are just one piece of the evolving puzzle in modern oncology. The future holds numerous opportunities for further exploration, refinements, and breakthroughs. Here are some areas where future innovations might emerge:
- Refinement of Combination Strategies: Researchers may explore other pairings of localized chemotherapy with different immunotherapeutic agents to enhance efficacy even further.
- Personalized Medicine: Advances in genomics and biomarker analysis could enable the fine tuning of treatment plans, ensuring that the right combination is selected for each individual patient.
- Drug Delivery Mechanisms: Building on the success of intravesical delivery, future work could investigate alternate methods for achieving sustained release of drugs in other tumor types.
- Real-World Evidence: As more data emerge from real-life clinical practice, treatment protocols can be continuously optimized, providing further clarity on the long-term benefits and potential challenges.
These prospects are not without their own set of challenging parts and knotty issues. However, the promise of improved patient outcomes, better safety profiles, and a broader range of therapeutic choices ensures that the oncology world is on the verge of a transformative era.
In Conclusion: A New Chapter in Bladder Cancer Treatment
The introduction of TAR-200 plus sacituzumab in the neoadjuvant setting marks a super important step forward in the management of muscle-invasive bladder cancer. For patients unable to undergo traditional cisplatin-based regimens, this combination offers hope in a landscape that has historically been laden with intimidating treatment options and overwhelming side effects.
The SunRISe-4 trial’s encouraging findings—demonstrating higher pathologic complete response rates, improved downstaging, and enhanced relapse-free survival—indicate that this dual approach may soon become a cornerstone of care for a challenging patient population. While there are still a few tangled issues and subtle details that need to be worked out, the overall trajectory is promising, offering both clinicians and patients a new way to steer through the twists and turns of bladder cancer treatment.
As we take a closer look at these developments, it is clear that the integration of localized chemotherapy delivery with systemic immunotherapy has the potential to significantly change the treatment paradigm. By reducing widespread toxicity, improving quality of life, and expanding the therapeutic arsenal for cisplatin-ineligible patients, this approach could redefine what effective cancer care looks like in the coming years.
Ultimately, the collaboration between researchers, clinicians, and patients will be key to realizing the full benefits of these innovative treatments. With continued investment in clinical trials, real-world data collection, and interdisciplinary collaboration, the future of bladder cancer care looks brighter than ever. It is our hope that these advancements will lead not only to longer survival but also to better overall experiences for patients navigating the sometimes confusing bits of cancer treatment.
Key Takeaways and Future Directions for Bladder Cancer Therapy
To summarize the main points discussed, here are the key takeaways from our review of TAR-200 plus sacituzumab therapy:
- The SunRISe-4 trial demonstrated a significant improvement in pathologic complete response and one-year relapse-free survival for patients treated with the combination therapy.
- The approach offers a promising alternative for cisplatin-ineligible patients, addressing several of the nerve-racking side effects and challenges associated with traditional chemotherapy.
- Localized drug delivery through intravesical administration reduces systemic toxicity, making it a super important option for enhancing patient comfort and outcomes.
- Integrating such novel therapies into current treatment paradigms requires careful consideration of patient selection, precise scheduling, and robust monitoring systems.
- Future advances may refine combination strategies further, incorporating personalized medicine and innovative drug delivery mechanisms to optimize outcomes.
As the oncology community continues to figure a path forward through the ever-changing landscape of cancer treatment, these insights will form the foundation for both clinical practice improvements and new research directions. The journey ahead promises to be full of opportunities to reimagine standard care practices and ultimately, to unlock better, more effective treatments for patients everywhere.
A Call to Action for Clinicians and Researchers
In closing, it is a super important moment for everyone involved in cancer care—from the treating physician to the dedicated pharmacist—to take note of these emerging treatment options. The evidence from the SunRISe-4 trial not only offers a viable alternative for patients who cannot undergo cisplatin-based therapy but also opens the conversation about embracing innovative, patient-tailored therapeutic strategies.
We encourage clinicians to:
- Keep abreast of the latest clinical trial data and treatment guidelines as this field evolves.
- Engage in interdisciplinary discussions and case conferences to share practical insights and overcome confusing bits together.
- Consider offering participation in clinical trials to eligible patients, thereby contributing to a broader understanding of these emerging therapies.
Similarly, researchers must continue to chip away at the remaining subtle details and hidden complexities through further studies, ensuring that these new therapies are continuously refined for maximum effectiveness and safety.
As we all work through the twists and turns of modern oncology, it is clear that collaboration, persistence, and innovation will be the bedrock upon which future breakthroughs are built. By embracing these changes and remaining vigilant in our pursuit of better outcomes, we can ensure that the future of bladder cancer care is not just about prolonging life but truly enhancing its quality.
Final Thoughts
The journey toward more effective and tolerable treatments is rarely straightforward. With new therapy combinations like TAR-200 plus sacituzumab, however, we are seeing a promising shift in how bladder cancer can be managed – especially for patients who previously had few options. While challenges remain, the adoption of innovative approaches that combine local and systemic therapies represents a tangible step forward in both efficacy and safety.
In this dynamic field, every new clinical insight contributes to a broader narrative of hope, resilience, and continued progress. By working together and staying focused on patient-centered care, we can transform the intimidating parts of cancer treatment into manageable, tailored strategies that truly make a difference in people’s lives.
Ultimately, the success of any new treatment is measured not just by improved clinical statistics but by the tangible improvement in the day-to-day lives of patients. As we move forward, let us keep the conversation open, the research vigorous, and the commitment to high-quality care unyielding. The evolving landscape of bladder cancer treatment is a testament to what can be achieved when science, medicine, and compassionate care come together to face even the most confusing bits and nerve-racking challenges head on.
Originally Post From https://www.pharmacytimes.com/view/tar-200-plus-anti-pd-1-therapy-shows-promising-neoadjuvant-activity-in-cisplatin-ineligible-muscle-invasive-bladder-cancer
Read more about this topic at
New treatment eliminates bladder cancer in 82% of patients
New hope for bladder cancer patients as breakthrough …