A New Era in Fighting KRAS-Driven Cancers
The landscape of cancer treatment is rapidly evolving, and a recent breakthrough in immunotherapy is poised to turn heads, especially when it comes to KRAS-driven pancreatic and colorectal cancers. Traditional therapies have long struggled with the tricky parts of battling these malignancies, but the novel vaccine ELI-002 2P is offering new hope where the stakes are extraordinarily high. As someone passionate about modern medicine and alternative approaches, I find this development both intriguing and promising—a true milestone in our fight against cancers riddled with tension and persistent challenges.
Recent research spearheaded by experts at the UCLA Health Jonsson Comprehensive Cancer Center has shown that this vaccine, designed to target the notorious KRAS mutations, can stimulate the immune system against a highly prevalent cancer driver. These exciting findings not only signal potential improvements in patient care but also highlight critical advances in immunotherapy and cancer vaccination.
KRAS Mutations in Pancreatic and Colorectal Cancers: Risks and Opportunities
KRAS mutations have been a particularly intimidating hurdle in oncology. Found in roughly 25% of solid tumors, these mutations are responsible for fueling nearly 90% of pancreatic cancers and 50% of colorectal cancers. For patients and clinicians alike, these statistics underscore just how formidable these cancers can be.
This breakthrough opens up the conversation around addressing the tangled issues that arise from these mutations. Traditional treatment methods often struggle to get around the confusing bits and awkward twists and turns of KRAS-driven cancers, leaving many patients facing recurring disease even after intensive treatment.
In my view, the study not only reaffirms the necessity for innovative therapies but also pushes us to get into a deeper discussion about targeting genetic mutations that have, until now, been challenging to treat. The ELI-002 2P vaccine exemplifies the need to tackle these cancers head-on and aims to clear the path toward more precise and long-lasting treatment outcomes.
Understanding the Science: How Does ELI-002 2P Work?
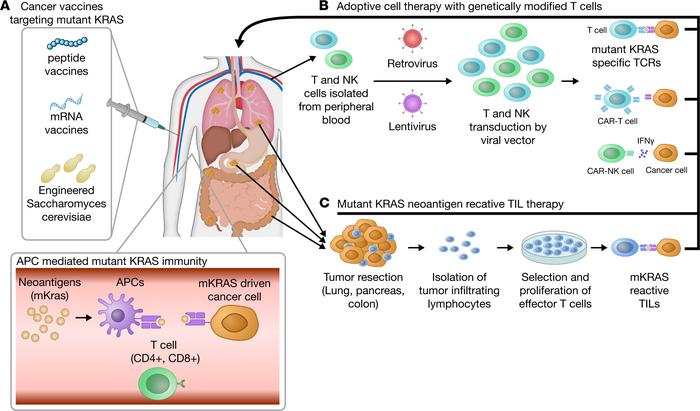
The science behind this novel vaccine is nothing short of fascinating. Unlike some highly personalized approaches that require a tailored vaccine for every patient, the “off-the-shelf” ELI-002 2P uses advanced amphiphile technology to directly shuttle tumor antigens into lymph nodes. This targeted delivery method encourages a powerful immune response by effectively training the body to recognize and eliminate cancer cells driven by KRAS mutations.
Let’s break down the key elements that make this vaccine a potential game-changer:
- Direct Lymph Node Targeting: By delivering antigens straight to the immune command center—the lymph nodes—the vaccine maximizes the body’s ability to mount a robust defense.
- Off-the-Shelf Consistency: Unlike personalized vaccines, ELI-002 2P is produced as a standardized product, making it readily available without the delays associated with customization.
- Dual Immune Response Activation: The vaccine stimulates both CD4+ helper T cells and CD8+ killer T cells. This dual approach not only enhances the immune response but also supports longer-lasting protection.
These insights into the innovative design of ELI-002 2P help us appreciate the vaccine’s multi-pronged approach to overcoming the nitty-gritty challenges associated with KRAS-driven cancers. It provides a more general, yet precise, method of harnessing the immune system to patrol and attack cancer cells—tackling those confusing bits associated with cancer recurrence head-on.
Patient Outcomes and the Impact of Strong Immune Responses
The early results from the extended 19.7-month follow-up are impressive. Patients who generated strong KRAS-specific T cell responses experienced a marked improvement in both relapse-free survival and overall survival. For instance, patients with robust immune responses saw their median relapse-free survival not reached, compared to a mere 3.02 months for those with lower responses—a difference that is both statistically and clinically significant.
These findings lead us to several key observations:
- Extended Relapse-Free Survival: Patients with a strong T cell response continued to remain free of disease far longer than anticipated, a critical consideration in cancers known for rapid recurrence.
- Overall Survival Benefits: The overall survival metrics exceeded historical norms, offering a glimmer of hope for those facing some of the most intimidating cancer types.
- Potential for Broader Anti-Tumor Activity: Interestingly, 67% of patients also developed immune responses against additional tumor-associated mutations, suggesting that the vaccine might trigger a more expansive attack on cancer cells than initially envisioned.
These observations are both exciting and critical for the future of cancer therapy. The strong evidence supporting the efficacy of this vaccine encourages further exploration and larger-scale clinical trials, aiming to replicate and extend these outcomes to benefit even more patients worldwide.
Immune System Activation: The Essential Role of T Cells
When interpreting these findings, it is important to understand the role of T cells in immune system activation and cancer defense. T cells are a fundamental component of the body’s immune surveillance system. By creating an environment where both CD4+ helper and CD8+ killer cells are activated, the vaccine ensures that the body is well prepared to tackle cancer cells in a focused manner.
Here are a few key factors regarding T cell responses:
- Longevity of Response: Many patients maintained their T cell responses over time, suggesting that these benefits could be durable.
- Correlation with Improved Survival: The presence of a strong T cell population directly correlated with longer relapse-free and overall survival, reinforcing the importance of robust immune activation.
- Dual Cell Activation: The coordinated response between helper and killer T cells is vital for a comprehensive defense against cancer cells.
This is something that we need to stress when discussing innovative treatment options. Immunotherapy, when working correctly, can rewire the body’s natural defenses to fight off cancer more effectively. What ELI-002 2P brings to the table is a method to exploit these subtle details of the immune system using advanced science, thereby countering one of the most overwhelming challenges in oncology.
The Broader Implications for Oncological Treatment
Beyond the immediate promising results in pancreatic and colorectal cancers, this study opens a door for a broader discussion on the treatment of KRAS-driven cancers. The traditional path—often marked by an overwhelming reliance on chemotherapy or radiation—has left many questioning whether a more targeted approach, like immunotherapy, could be the key to improved long-term outcomes.
Here are several points to consider in the broader context of oncological treatment:
- Standardization vs. Personalization: One of the standout features of this vaccine is its “off-the-shelf” nature. Unlike the nerve-racking process of personalizing treatments for each individual patient, a standardized vaccine can be both more accessible and more efficient.
- Cost Efficiency and Simplicity: The production of personalized vaccines can be prohibitively expensive and time-consuming. This vaccine, by contrast, is designed for broader application, potentially reducing costs and simplifying treatment logistics.
- Future Research Opportunities: The promising initial results warrant larger Phase 2 studies and beyond. Advancements in this area might eventually lead to similar standardized solutions for other genetic mutations driving cancer.
The potential impact on future patient care cannot be understated. The prospect of using a consistent, generalized immune-boosting agent against one of the most elusive cancer drivers is truly exciting. If further trials confirm these early findings, we could witness a paradigm shift in how we address some of the most snake-hearted challenges in cancer treatment.
Comparing the Vaccine Approach with Other Cancer Therapies
When we take a closer look at the landscape of cancer therapies, especially those aimed at KRAS-driven cancers, there are significant differences between traditional approaches and this innovative vaccine therapy. Traditional treatments, like chemotherapy, often come with a range of side effects and require navigating through complicated pieces of treatment protocols. Meanwhile, the vaccine offers a more targeted and refined way to get the immune system to attack cancer cells.
Comparative highlights include:
| Treatment Approach | Pros | Cons |
|---|---|---|
| Chemotherapy |
|
|
| Personalized Vaccines |
|
|
| ELI-002 2P Vaccine |
|
|
This table illustrates that while every approach has its benefits and drawbacks, the ELI-002 2P vaccine stands out by offering an easier method to steer through the maze of cancer treatment. It sidesteps some of the nerve-racking steps required in personalizing treatments, instead offering a broadly applicable tool that could one day reduce the complexity of care for patients battling these aggressive cancers.
Working Through the Tricky Parts of Immune Activation and Safety
While the early results of the vaccine have been promising, it is important to recognize that, like all innovative treatments, there are still some twisted challenges to address. The safety and efficacy data reviewed in the context of the AMPLIFY 201 Phase 1 trial serve as an essential reminder that rigorous clinical testing remains key to integrating new therapies into everyday practice.
Some of the critical points to consider include:
- Monitoring Immune Responses: Robust and sustained T cell activation is at the heart of this vaccine’s success. Ongoing studies will need to continue assessing how long these responses are maintained and whether they translate into long-term remission for a greater proportion of patients.
- Safety Profile: Although early results point to a favorable safety profile, longer follow-ups and larger trials are necessary to rule out any delayed adverse effects that could be linked to immune system over activation.
- Expansion Into Broader Patient Populations: Current trials have focused on patients with minimal residual disease post-surgery. Future studies should aim to understand the vaccine’s effectiveness in patients at different stages of disease progression.
These factors are all super important when assessing any new treatment. It’s not enough to just see improved survival rates; we must also ensure that the method of activating the immune system does not lead to unintended consequences. The trial’s results, while inspiring, remind us that further research and cautious optimism are needed before this approach can become a standard in oncological treatments.
Expert Perspectives and Future Research Directions
In the words of Dr. Zev Wainberg—one of the key figures behind the study—the results are indeed a breakthrough, particularly for patients with recurring or hard-to-treat cancers. Observations that those who mounted a strong immune response experienced dramatically better outcomes strongly point towards a new era where immunotherapy takes center stage in cancer treatment strategies.
Experts in the field agree that the full potential of a vaccine like ELI-002 2P will become more evident as research deepens. Some of the avenues for future studies include:
- Expanding Phase 2 Trials: A larger patient population could provide more robust data and help solidify the vaccine’s role in the treatment paradigm.
- Investigating Combination Therapies: Future research might explore how this vaccine could be used alongside other treatments such as checkpoint inhibitors or traditional chemotherapy, potentially creating synergistic effects against cancer cells.
- Exploring Its Use in Other KRAS-Driven Cancers: Given that KRAS mutations drive many solid tumors, it will be crucial to examine whether similar immune responses can be elicited across other cancer types.
When we take a closer look at the subtleties of immune activation and cancer treatment, we see that innovations like the ELI-002 2P vaccine act as a catalyst, encouraging researchers to explore adjacent fields and rework our approach to cancer therapy. The promise of a standardized vaccine that can handle the nerve-racking issues of personalized treatments lights a path toward a future where managing cancer becomes less overwhelming and more predictable.
Integrating Alternative Approaches and Holistic Perspectives
While modern medicine and targeted immunotherapies are carving out new paths in cancer treatment, there is also growing interest in how alternative therapies and nutritional interventions might complement these breakthroughs. Though far from being a replacement for clinical interventions, holistic approaches can be super important in addressing the overall well-being of patients.
For instance, some complementary strategies include:
- Diet and Nutrition: Certain diets rich in antioxidants and anti-inflammatory foods might support immune function, potentially enhancing the body’s response to treatments like ELI-002 2P.
- Mind-Body Interventions: Practices such as meditation and yoga can alleviate stress, which is often a hidden complexity in the recovery and healing process.
- Supplemental Therapies: While more research is needed, supplements known to support immune health may serve as adjuncts to boost treatment outcomes.
Although these approaches have their own set of tricky parts and require careful consideration, the goal is to craft a treatment environment that not only attacks the cancer directly but also supports the patient’s overall resilience. In this context, emerging therapies like the KRAS-targeted vaccine represent one piece of a broader, holistic puzzle aimed at managing and eventually overcoming cancer.
Patient Perspectives and the Real-World Impact
No discussion about advancing cancer treatments is complete without considering the patient perspective. For individuals facing a diagnosis of pancreatic or colorectal cancer, the news of a vaccine that can effectively spur the immune system to fight back offers a ray of hope amid a landscape that can feel overwhelmingly off-putting.
Patients have long been caught in the crossfire of treatments that often leave them facing a litany of side effects and a nerve-racking sense of uncertainty over recurrences. The idea that a standardized vaccine could significantly extend relapse-free survival—and perhaps even overall survival—changes the narrative. It transforms the cancer journey from one of sheer survival to one where improved quality of life and extended periods of remission become attainable goals.
Furthermore, the affordable and accessible nature of an off-the-shelf vaccine eases the burden on healthcare systems and patients alike. By cutting down on the expensive and time-consuming process of creating personalized vaccines, this approach could streamline treatment and make it available to a wider segment of the population, effectively democratizing advanced cancer care.
Taking the Wheel: Challenges and the Road Ahead
While the promise of the ELI-002 2P vaccine is undeniable, it is important to remember that the pathway to widespread use is not without its nerve-racking challenges. As with any emerging therapy, long-term studies are needed to confirm both its efficacy and safety over extended periods.
Some of the hurdles that remain include:
- Long-Term Efficacy and Safety: Ensuring that the immune responses remain robust while avoiding potential autoimmune complications requires careful, ongoing observation.
- Adaptability to Diverse Populations: Future studies should explore how various demographics respond to the vaccine, given that genetic variability and overall health status can influence outcomes.
- Integration with Existing Therapies: Researchers must figure a path that allows the vaccine to be combined seamlessly with other treatment protocols, whether it’s chemotherapy, radiotherapy, or other immunotherapy regimens.
Overcoming these nerve-racking hurdles will require concerted efforts from clinicians, researchers, and regulatory authorities. The early success of the trial is encouraging, but the twists and turns of cancer treatment demand that every new approach is scrutinized carefully to ensure it truly benefits patients in the long run.
The Promise of Next-Generation Vaccines
Looking ahead, researchers are already setting their sights on developing next-generation versions of this vaccine. The new ELI-002 7P vaccine, designed to target an even broader spectrum of KRAS mutations, represents an exciting expansion of this therapeutic strategy.
This next step carries several potential advantages:
- Broader Mutation Coverage: By addressing a wider array of KRAS mutations, the new vaccine could offer protection for a larger number of patients.
- Enhanced Immune Activation: Building on the solid foundation of its predecessor, the next-generation vaccine may stimulate an even more robust immune response, leading to improved outcomes.
- Streamlined Administration: Maintaining the off-the-shelf advantage, it promises a treatment that is consistent, easy to deploy, and less resource-intensive than personalized therapies.
As clinical trials expand and more real-world data come in, the impact of these vaccines on patient survival and quality of life will become clearer. The progress made so far is a testament to the power of scientific innovation and the persistent drive to get around the confusing bits of cancer treatment and improve patient outcomes.
Final Thoughts: A Step Toward a More Optimistic Future
The emergence of the KRAS-targeted vaccine ELI-002 2P marks a significant turning point in the battle against some of the most intimidating forms of cancer. While there remain many tangled issues to address—ranging from long-term safety to broader applicability—the early clinical results are a cause for cautious optimism.
This innovative approach demonstrates that by combining advanced science with a deep understanding of the body’s own immune defenses, we can begin to steer through the challenges posed by cancers that have long been considered a tough nut to crack.
Of course, this is only the beginning. As larger-scale studies progress and next-generation vaccines become available, we are likely to see further refinements and improvements. These advancements will not only reduce the complicated pieces associated with personalized treatment approaches but also offer a more standardized, accessible form of care that could revolutionize how we manage KRAS-driven cancers.
For patients and healthcare professionals alike, the road ahead is filled with promise. With careful, ongoing research and a commitment to patient safety, this breakthrough could very well reshape the future of oncological treatment, offering longer remissions and improved quality of life. It is a reminder of the power of science to overcome the small twists and hidden complexities of cancer and drive us toward a more hopeful future.
In conclusion, while the journey is not without its challenges, the potential of this new vaccine to extend and improve lives marks a significant milestone in modern medicine. It is essential for us to celebrate such achievements while remaining mindful of the work that still needs to be done. With continued research, collaborative efforts, and an unwavering commitment to patient care, we are on the cusp of a new era in the fight against cancer—an era where the immune system can be precisely and effectively mobilized to tip the scales in favor of life.
As we reflect on this exciting development, there is a compelling need for more research funding, broader clinical trials, and an open-minded approach to integrating innovative therapies into everyday practice. Only then can we truly claim to have taken a major step toward eradicating some of the most intimidating challenges in cancer care.
Ultimately, the promise of targeted immunotherapy—embodied in the ELI-002 2P vaccine—provides a reason for hope and a clear message: even in the face of cancers loaded with issues and stubbornly resistant to conventional treatments, there is a pathway to more effective, longer-lasting, and less nerve-racking therapies. As we continue to get into the hidden complexities of cancer and harness the power of the immune system, the future looks decidedly more hopeful for those affected by these aggressive diseases.
In sharing these insights, I invite my fellow healthcare professionals, researchers, and policy makers to think critically about the directions our treatment protocols can take. Let us continue to work through the tricky parts, address every fine detail, and spur further innovation that could one day transform cancer treatment beyond recognition.
Originally Post From https://www.news-medical.net/news/20250812/Novel-vaccine-shows-promise-against-KRAS-driven-pancreatic-and-colorectal-cancers.aspx
Read more about this topic at
Innovative directions in immunotherapy research
Advances in Immunotherapy and Innovative Therapeutic …