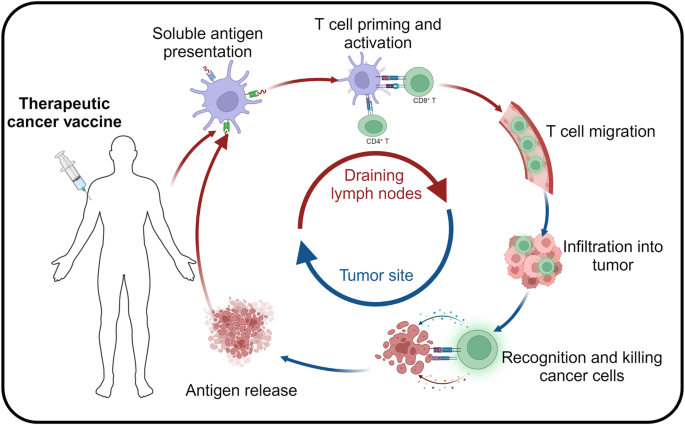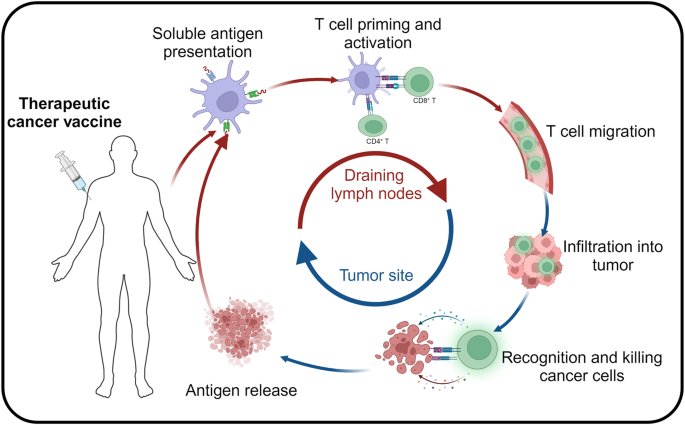
Introduction: A New Chapter in Cancer Immunotherapy Research
The recent news about IO Biotech’s phase 3 trial for a melanoma cancer vaccine has sparked widespread discussion in the medical research community. Although the trial “narrowly” missed the statistical significance needed for its primary endpoint, many experts remain optimistic about the potential of IO’s therapeutic approach. In this opinion editorial, we take a closer look at the trial’s design, the government’s regulatory expectations, and the wider implications for patients and oncologists alike. We will also explore how this study fits into the broader trends in cancer immunotherapy and discuss the challenges, twisted parts, and subtle details that continue to shape this evolving field.
At first glance, the results may seem like a setback; however, a detailed analysis reveals that there is more to this story than meets the eye. The trial’s data have left researchers with important questions about the future direction of cancer vaccines, especially those designed to work in tandem with standard therapies like Keytruda. The discussion below dives into the many facets of the study, including trial design, data interpretation, regulatory hurdles, and the broader implications for oncology practice.
Evaluating the Melanoma Trial Data: The Fine Points of Progression-Free Survival
At the heart of the debate lies the trial’s primary endpoint: progression-free survival (PFS). In the global trial involving 407 patients, the combination treatment of IO Biotech’s Cylembio with Keytruda was compared to Keytruda alone. While the median PFS reached 19.4 months with the combination versus 11 months with Keytruda alone, many in the research community are keenly aware of the subtle points that come along with such data.
The trial data highlighted an “early and sustained separation of PFS curves” between the two treatment arms. However, the hazard ratio of 0.77 was associated with a P value of 0.056, just missing the traditional threshold for statistical significance. These results have naturally raised a series of questions:
- What does a P value of 0.056 mean for patients and clinicians?
- How do we interpret a near-significant statistic when the absolute difference in PFS is substantial?
- Can further analyses help shed more light on the potential benefits of this therapeutic approach?
While some find the result to be disappointing, others believe that the data—even in their near-miss—point to a promising direction for immunotherapy. In particular, a post-hoc analysis of the 371 patients who had not received prior anti-PD-1 treatment showed a nominal P value of 0.037, reinforcing the potential clinical advantage in a well-defined patient subgroup.
Understanding the Implications of a “Narrow” Statistical Miss
Many critics have pointed to the “narrow” miss as evidence that the trial might have been inherently risky or possibly underpowered. However, it is important to remember that the fields of oncology and immunotherapy are laden with tricky parts and tangled issues. In this context, a narrowly missed statistical threshold does not necessarily mean the treatment is ineffective.
The trial’s outcome underscores a key concept in clinical research – that small distinctions in patient outcomes can sometimes be lost in the noise of broader statistical analyses. The almost significant improvement in progression-free survival suggests that for some subgroups of patients, the vaccine combination could offer an improved disease control option. The nuanced findings remind us that in the world of cancer research, one must often dig into the data, examine the small twists, and appreciate that not every benefit is immediately visible in conventional outcome measures.
Challenging Regulatory Expectations: Preparing to Meet the FDA
Despite the trial’s mixed results, IO Biotech remains steadfast in their approach to moving forward with discussions with the FDA. The company plans to engage with regulatory bodies later this year to discuss the totality of the data and explore submission pathways for potential approval. This proactive stance reflects a growing recognition that government regulators sometimes consider a broader spectrum of evidence beyond the strict p value threshold.
In preparing to meet the FDA, IO Biotech is stepping into a nerve-racking but essential conversation about how to define meaningful clinical benefits. Here are some regulatory considerations that point to the challenges and subtle details in such discussions:
- Holistic Data Evaluation: FDA guidelines are increasingly open to considering a wide range of data – including secondary endpoints, subgroup analyses, and trends in overall survival.
- Risk-Benefit Balance: Even if the statistical breakthrough is not perfect, a treatment that shows significant improvement in quality of life and disease control can be seen as a valuable option for patients with few alternatives.
- Innovative Trial Designs: The industry is gradually embracing more flexible trial designs that capture the intricate pieces of patient response, paving the way for novel therapies to be approved on a different evidence basis.
This situation highlights an off-putting but common reality – the regulatory environment is as much an art as it is a science. Navigating through these regulatory waters means balancing numerical results with the overall clinical picture and adjusting expectations accordingly.
Combination Therapeutics: The Different Strategy of Off-the-Shelf Vaccines
Unlike the highly personalized cancer vaccines being developed by mRNA giants like Moderna and BioNTech, IO Biotech’s approach with Cylembio represents a distinct strategy. Cylembio is derived from a platform known as T-win, which uses off-the-shelf vaccines that aim to stimulate the patient’s own T cells within the tumor microenvironment. This method is seen as a more scalable option, designed to overcome some of the challenges associated with individualized vaccine production.
Here are some of the key differentiators of this approach:
- Ease of Production: Off-the-shelf vaccines are generally less complicated to manufacture and distribute compared to personalized treatments that require custom formulations.
- Combination Versatility: When combined with an approved therapy such as Keytruda, these vaccines might create synergistic effects that magnify the immune response.
- Cost-efficiency: Scaled manufacturing processes can potentially reduce costs, making advanced cancer treatments more accessible to a broad range of patients.
This innovative approach, though not without its own set of confusing bits and taste of risk, offers a glimpse into how the landscape of cancer vaccine research is evolving. As funding and interest in therapeutic cancer vaccines continue to surge, it becomes increasingly critical to consider not only the statistical outcomes but also the practical advantages of such combination therapies in a real-world setting.
Statistical Analysis: Decoding the Data Behind the Headlines
A closer look at the trial data helps us understand why many experts remain cautiously optimistic. The trial’s primary endpoint, progression-free survival, is a key metric in oncology that indicates how long a patient lives without the cancer advancing. Although the P-value of 0.056 for the full study cohort might seem discouraging, the following table summarizes the main findings:
| Parameter | Combination (Cylembio + Keytruda) | Keytruda Alone |
|---|---|---|
| Median PFS (months) | 19.4 | 11 |
| Hazard Ratio | 0.77 | |
| P Value (Overall) | 0.056 | |
| Subgroup P Value (no prior anti-PD-1) | 0.037 | |
This table lays out the deceptively simple numerical differences that, upon close examination, reveal many of the trial’s hidden complexities. The improvement in median PFS suggests that the combination approach may delay disease progression significantly. At the same time, the intricate debate over statistical significance highlights the challenges in interpreting such data, especially when slight differences in methodology or subgroup selection can mean the difference between a clear success and a near-miss.
Interpreting the Overall Survival Trends: A Closer Look at Future Promise
While progression-free survival was the primary focus, the trial also touched upon overall survival (OS) trends. Although the OS data are still immature, early observations point to a promising trend favored by the combination therapy. IO Biotech’s Chief Medical Officer, Dr. Qasim Ahmad, noted that there appeared to be an improvement in OS across almost all patient subgroups. Such trends are key when it comes to interpreting the long-term benefits of a new treatment.
It is essential to keep in mind that overall survival is often laden with many complicating pieces including post-progression treatments and patient demographics. Once more mature data become available over the next six to nine months, the field will likely gain a clearer picture of the treatment’s impact on patient longevity. At its core, the dialogue about OS reiterates that success in cancer therapy is measured not only by immediate response rates but also by the sustained benefit over time.
Weighing the Promises Against the Limitations: A Balanced Perspective
When scrutinizing such trials, both patients and the medical community must manage their way through a raft of competing factors. The benefits observed in progression-free survival and overall survival trends are encouraging, yet they come with certain important caveats:
- Sample Size Considerations: With 407 patients included in this global trial, the numbers are promising, but further studies in larger or more diverse populations may be necessary to reduce uncertainty.
- Subgroup Variability: The post-hoc analysis showing more significant results in patients without prior anti-PD-1 treatment suggests that patient selection criteria will be critical in future trials.
- Follow-Up Duration: Mature overall survival data, which remain to be seen, could either bolster or question the early findings.
- Combination Therapy Nuances: The potential additive or synergistic effects of combining therapies such as a vaccine and an anti-PD-1 treatment introduce layers of complexity in understanding the true benefit.
This balanced perspective highlights a broader truth about oncological research—every promising result is accompanied by a few confusing bits and multiple areas needing further exploration. For clinicians and patients alike, this means staying informed and expecting a period of adjustment as new data become available.
Patient Perspectives: What Does This Mean for Those Battling Melanoma?
For patients battling advanced melanoma, these developments represent both hope and uncertainty. On one hand, the nearly doubled progression-free survival period is a super important outcome that can translate into more quality time and potentially better quality of life. On the other hand, the trial’s near miss in meeting statistical significance leaves open questions about the consistency and reliability of the benefits observed.
Patients considering participation in similar clinical trials or those evaluating treatment options should keep the following points in mind:
- Proactive Consultation: Discuss the details and potential benefits of combination immunotherapies with your oncologist. Ask specifically about the advantages and possible risks of integrating a cancer vaccine with standard treatment.
- Staying Informed: As further data emerge—especially regarding overall survival—the understanding of these treatments will continue to evolve. Keeping up with the latest research developments can help in making informed decisions.
- Individual Factors: Each patient’s medical history, prior treatments, and overall health play a key role in determining the suitability of a therapy.
- Quality of Life Considerations: Beyond the raw numbers, it is important that treatment decisions also factor in potential improvements in day-to-day living and overall well-being.
This patient-focused view underscores that while numerical endpoints are critical, the ultimate goal of any cancer treatment is to positively impact lives. Maintaining hope, while carefully weighing the advantages against the potential challenges, can help patients and caregivers find the best path forward in an ever-evolving landscape.
Expert Opinions: Voices from the Oncology and Immunotherapy Fields
Across the academic and clinical worlds, there is a recurring sentiment that cautious optimism is warranted. Experts point to several key factors that continue to drive excitement about immunotherapy, especially combination approaches that incorporate cancer vaccines:
- Diverse Mechanisms of Action: The idea of employing off-the-shelf vaccines to stimulate T cells in the tumor microenvironment represents a creative departure from highly personalized therapies. This diversification in treatment methods can lead to breakthroughs in cases where conventional therapies fall short.
- Real-World Applicability: Off-the-shelf approaches may not only cut costs but also streamline the process of treatment delivery. This could potentially allow therapy to reach more patients, addressing some of the time-consuming aspects of personalized medicine.
- Robust Safety Profiles: The early observations that the combination of Cylembio with Keytruda does not introduce significant additional systemic toxicity are very encouraging. In a field where treatment tolerability is essential, minimizing adverse effects is key to a therapy’s overall appeal.
- Evolving Clinical Endpoints: As regulatory bodies re-evaluate what truly matters in clinical outcomes, factors such as quality of life, long-term survival, and patient satisfaction are increasingly considered alongside traditional statistical measures.
These perspectives remind us that the immune system’s interactions with cancer remain a topic full of twists and turns. While no treatment is free of risk, understanding the multiple dimensions of a therapy helps clinicians and researchers figure a path toward the best possible patient outcomes.
Lessons Learned: Working Through the Trial’s Subtle Details
The lessons drawn from the IO Biotech trial are manifold. There are several tangled issues and little details that researchers and clinicians must carefully consider as they work on improving cancer vaccines:
- Trial Design Improvements: Future studies might benefit from refined patient stratification to better capture those most likely to respond. For instance, identifying biomarkers or clinical signatures could help isolate the subgroup that derives the maximum benefit from the combination approach.
- Adaptive Endpoints: Rethinking how endpoints such as progression-free survival and overall survival are used in clinical decision-making might provide a more complete picture of treatment efficacy. Adaptive trial designs that incorporate interim analyses and flexible endpoints could prove advantageous.
- Cross-disciplinary Collaboration: The complexity of combining immunotherapies with established treatments calls for collaborative approaches that bring together oncologists, immunologists, statisticians, and regulatory experts.
By paying close attention to these fine points and working through the many small twists inherent in cancer research, the industry can continue to refine its strategies and design better, more effective clinical trials. This adaptive approach is critical as the landscape of oncology moves towards more personalized and targeted care.
Future Directions: How Will Cancer Vaccines Shape the Oncology Landscape?
The current study represents a stepping stone rather than a final destination. As researchers continue to explore the potential of combination therapies, several emerging trends may soon redefine the path of cancer treatment:
- Integration of Novel Biomarkers: Using innovative biomarkers to predict which patients might benefit most from a combination of therapies can transform clinical decision-making. These biomarkers, once validated, could guide treatment choices and optimize outcomes.
- Enhanced Trial Technologies: Advances in imaging, genomic profiling, and real-time data analysis are already beginning to influence trial designs. With better technology to poke around the interplay between different therapies, clinical studies will likely yield more precise data.
- Collaborative Research Initiatives: Global collaborations between academia, industry, and regulatory bodies will remain critical. Sharing data and insights across multiple centers can help mitigate the challenges of small sample sizes and heterogeneous patient populations.
- Patient-Centric Models: As patients become more informed and involved in their treatment options, clinical trials will need to reflect real-world concerns, balancing efficacy with quality of life aspects. This means that patient-reported outcomes and satisfaction measures may soon play a more central role in therapeutic assessments.
Charting the future path for cancer vaccines will require both optimism and realism. While the promise of improved immune responses and longer periods without disease progression is appealing, researchers must remain grounded in the understanding that progress in this field is full of competing factors, each with its own set of challenges and unforeseen complications.
Looking Ahead: The Path Forward Amid Uncertainty
There is no denying that the journey ahead is both exhilarating and intimidating. For IO Biotech and similar companies working in the realm of cancer vaccines, every trial is a learning opportunity. The current data, with its near-miss of statistical significance, is a reminder that even promising therapies can encounter unexpected twists and turns.
Stakeholders—from researchers and clinicians to patients and regulators—must find their way through the challenging parts of this journey by:
- Maintaining an open mind about the potential of new combination therapies.
- Supporting ongoing research that refines patient selection and trial methodologies.
- Advocating for regulatory frameworks that value the totality of clinical evidence.
- Prioritizing both clinical efficacy and quality of life in treatment development.
It is in these efforts that the hope for a breakthrough in immunotherapy truly lies. While the current trial results may seem like a mixed bag, they also serve as an invitation to the scientific community to dig into the data, take a closer look at the subtle parts, and stay committed to the cause of improving patient care.
Conclusion: Finding a Path Through the Twists and Turns of Cancer Research
The evolution of cancer immunotherapy remains one of the most dynamic areas in modern medicine. IO Biotech’s recent phase 3 trial for its melanoma cancer vaccine, despite narrowly missing a key statistical benchmark, has contributed valuable insights to the field. The data suggest that, for certain patient groups, the combination of Cylembio and Keytruda could meaningfully extend progression-free survival and potentially overall survival.
As we reflect on these findings, we are reminded that the true measure of progress in cancer research is not solely about achieving perfect numerical outcomes. It is about consistently striving to make small improvements that, when taken together, pave the way toward better treatment options and improved quality of life for patients.
Many of the challenges in evaluating these new therapies are laden with confusing bits and tangled issues that require careful dissection. The statistical nuances, regulatory hurdles, and technological innovations represent both obstacles and opportunities. With every new study, the field moves one step closer to unlocking the full potential of the immune system in the battle against cancer.
Ultimately, the discussion sparked by this nearly successful trial is emblematic of the broader journey in oncology: a journey defined by incremental achievements, informed debates, and a shared commitment to enhancing patient care. As experts continue to figure a path through the regulatory maze and as future trials refine our understanding of combination immunotherapies, there is a collective sense that what may seem like a setback today could very well be the foundation for tomorrow’s breakthroughs.
For patients, clinicians, and researchers alike, the message is clear: persistence in the face of uncertainty, along with continued collaboration and innovation, remains the key to overcoming the many challenges currently facing cancer immunotherapy. While the road ahead is dotted with intimidating obstacles, it is also illuminated by the promise of transformative advancements that have the power to save lives and redefine what is possible in cancer treatment.
In the end, the evolution of cancer vaccines, as exemplified by the IO Biotech trial, is a testament to the resilience and adaptability inherent in the field of oncology. As we continue to learn from both successes and near-misses, the hope is that every bit of data – every subtle detail – will contribute to a future where cancer is not an insurmountable foe, but rather a challenge that can be effectively managed through innovative and patient-centric therapies.
The journey is far from over, and the dialogue sparked by this trial serves as a constructive reminder that progress in medicine is a marathon, not a sprint. With continued research, collaborative efforts, and an unwavering commitment to patient outcomes, the future of cancer immunotherapy looks set to advance in leaps and bounds despite the occasional statistical hiccup. The path may seem fraught with tangled issues and intimidating regulatory twists and turns, but it is one filled with promise, potential, and the enduring spirit of scientific discovery.
As we look ahead, the challenge for all stakeholders will be to remain focused on the bigger picture—balancing statistical data with real-world impact, and working through every confusing bit with an eye toward meaningful progress. In the intricate dance of innovation and regulation, every small step matters, and each new insight brings us closer to a world where effective cancer treatments are within reach for everyone.
In summary, IO Biotech’s latest trial, with all its successes and shortcomings, provides a valuable case study for the oncology community. It reinforces the idea that statistical outcomes, while important, are just one piece of a much larger puzzle in the fight against cancer. By taking a holistic approach—one that considers the subtleties of patient response, the variability of clinical endpoints, and the dynamic interplay of novel treatment methods—we can continue to move forward and ultimately find a viable path to improving outcomes for advanced melanoma patients.
The future of cancer immunotherapy is bright, but it requires all of us—researchers, clinicians, regulators, and patients—to work together, stay informed, and maintain the drive to explore every promising avenue. As we continue to chart this exciting, if sometimes nerve-racking, frontier in medicine, the lessons learned today will serve as the building blocks for the breakthroughs of tomorrow.
Originally Post From https://www.fiercebiotech.com/biotech/cancer-vaccines-narrow-phase-3-fail-wont-stop-io-heading-fda
Read more about this topic at
Using Optimism to Overcome Adversity
Intentional Optimism: Finding Purpose in Every Setback