Assessing Ivermectin: A New Frontier in Cancer Therapy
The world of cancer treatment is constantly evolving, with researchers regularly poking around new ways to beat some of the most intimidating forms of the disease. One emerging area of interest involves ivermectin – originally an approved antiparasitic medication – and its potential role when combined with modern immunotherapy. This editorial takes a closer look at this evolving research trend, the science behind it, and whether repurposing this well-known drug might be a game changer for patients suffering from aggressive cancers.
From Antiparasitic Agent to Oncology Innovator
Ivermectin initially made headlines for revolutionizing the management of parasitic infections such as river blindness, lymphatic filariasis, and strongyloidiasis during the 1980s. Over the decades, its safety profile and cost-effectiveness have made it a staple in global health. Now, emerging laboratory and preclinical studies suggest that ivermectin might offer a new benefit: helping to slow down cancer cell growth and even promote their death. Researchers are now diving in to explore its possible synergy with modern immunotherapeutic agents.
Understanding Ivermectin’s Mechanism in Cancer Treatment
When we take a closer look at ivermectin and its potential anticancer properties, there are several tricky parts to consider. Laboratory studies indicate that the drug operates on multiple fronts:
- Blocking Tumor Growth Pathways: Evidence suggests that ivermectin may interfere with pathways such as PAK1 and WNT-TCF, both of which are involved in cancer proliferation and metastasis.
- Inducing Programmed Cell Death: The drug can cause mitochondrial dysfunction in cancer cells, potentially leading to programmed cell death or apoptosis.
- Modifying the Tumor Microenvironment: Preliminary studies indicate that ivermectin might reduce the population of cancer stem-like cells, making tumors more visible to the immune system.
- Boosting Immunotherapy Effects: Early models point to a potential enhancement of immune checkpoint inhibitors – a cornerstone of modern oncological treatment – which could lead to improved outcomes for patients with difficult-to-treat cancers.
While the pathway from antiparasitic to anticancer agent is rife with twisted issues and hidden complexities, these initial findings are promising enough to encourage further clinical investigation.
The Ongoing Clinical Trial: Ivermectin and Immunotherapy for Metastatic Triple-Negative Breast Cancer
One of the most nerve-racking subtypes of breast cancer is triple-negative breast cancer (TNBC). TNBC is known for its early relapse, limited treatment choices, and aggressive behavior. Despite the progress made through immune-based therapies like pembrolizumab, many patients with metastatic TNBC still face overwhelming challenges once their disease progresses after initial therapies.
In this context, the ongoing phase I/II study seeks to combine the off-label use of ivermectin with established immunotherapy medications – specifically balstilimab or pembrolizumab – to see if this duo can improve patient outcomes. The study is innovatively designed to assess safety, tolerability, and potential efficacy in these patients.
Trial Details: A Snapshot
| Aspect | Description |
|---|---|
| Trial Type | Phase I/II, single-arm, interventional, open-label |
| Participants | 34 adults with histologically confirmed advanced metastatic TNBC |
| Performance Status | ECOG 0-1 (indicating a relatively healthy performance capacity) |
| Study Location | Cedars-Sinai Medical Center, Los Angeles, USA |
| Start Date | October 13, 2023 |
| Estimated Completion | October 2026 |
| Treatment Protocol |
|
| Eligibility |
|
This trial is a prime example of how scientists and clinicians are trying to work through the fine points of repurposing drugs, as they get around the challenging bits in traditional cancer treatment options. The study design is focused on discovering whether combining ivermectin with immune checkpoint inhibitors can produce a meaningful clinical benefit.
Key Elements of the Treatment Protocol and Their Rationale
The trial’s treatment protocol is designed to maximize the potential interaction between ivermectin and the immunotherapy agents. The dosing schedule ensures that both drugs are administered in a coordinated manner to tackle cancer cells on several fronts at once. Here are some of the crucial details:
- Ivermectin Dosing: Administered on specific days within each treatment cycle, allowing the drug’s anticancer properties to build up gradually.
- Immunotherapy Dosing: Administered via intravenous infusion shortly after ivermectin, these drugs aim to boost the patient’s immune response against tumor cells.
- Cycle Duration: With cycles repeating every 21 days and treatment potentially extending to approximately two years, the study offers a long horizon to monitor efficacy and safety.
One of the appealing aspects of this study is that it builds on previous knowledge of each drug. Ivermectin’s well-established safety profile makes it a candidate for repurposing, reducing some of the risk factors associated with introducing entirely new medications. Still, combining it with novel immune agents introduces its own set of tricky parts – making it a subject for careful observation.
Efficacy and Safety: Balancing Promise and Risk
One of the largest challenges in refining cancer treatment is figuring a path that balances efficacy with minimal toxicity. In the case of repurposing ivermectin, there is both optimism and caution. On one hand, preclinical studies provide a promising signal that ivermectin might enhance the effects of checkpoint inhibitors – drugs that have already transformed the outlook for many cancer patients.
However, the combination isn’t free from concerns. Some of the intimidating and complicated pieces include:
- Potential drug interactions that could modify the way ivermectin affects both healthy and cancerous cells.
- Possible side effects when combining an antiparasitic drug with immunotherapy, which already carries its own spectrum of adverse events.
- The challenge of determining the optimal dosing schedule that minimizes toxicity while maximizing anticancer effects.
Thus, the trial does more than just measure tumor shrinkage; it also carefully evaluates tolerability and monitors for any unexpected negative outcomes, ensuring that the overall benefit-risk balance is judged fairly.
Deciphering the Clinical Rationale: Why Immune Checkpoints and Ivermectin?
At the heart of this research is the aspiration to make immunotherapy more effective, especially in cancers known for a knack for evading the immune system. The rationale considers several tricky issues:
- Checkpoint Inhibitor Resistance: Not every patient responds sufficiently to PD-1 or PD-L1 inhibitors. The addition of ivermectin is hypothesized to push the immune system harder against tumors.
- Tumor Growth Pathway Inhibition: Ivermectin’s potential to block PAK1 and WNT-TCF signaling helps chip away at the tumor’s internal survival strategies, thus making the tumor more vulnerable.
- Enhancing Immune Recognition: By modifying the tumor microenvironment, ivermectin might help the body’s immune cells better detect and target malignant cells.
Combining these effects could lead to a scenario where immunotherapy not only gets a stronger foothold, but also acts in conjunction with ivermectin to target cancer cells more effectively. These ideas are still in the early stages, and the ongoing trial is both exciting and nerve-racking as it could lead to new strategies in managing aggressive cancers.
Expanding the Horizon: Ivermectin Beyond TNBC
While the current clinical trial is focused on metastatic triple-negative breast cancer, similar strategies might be applicable to other stubborn forms of cancer. The concept of repurposing is gaining traction in the medical community due to several key reasons:
- Established Safety Profiles: Drugs like ivermectin already have a long track record, reducing the barriers associated with clinical safety testing.
- Cost-Effectiveness: Compared to newer, custom-designed cancer drugs, repurposed medications often come with lower development costs and faster regulatory approvals.
- Broader Application: If successful in TNBC, similar clinical studies can be launched to examine potential benefits in other cancers, including colorectal, lung, and prostate cancers, each with its own set of challenging twists and turns.
However, embracing repurposing is not without its own set of tangled issues. Each cancer type is loaded with its own problems, and figuring a path to success will require adapting the treatment strategies based on the fine points of tumor biology and the patient’s overall condition.
Tackling Public Skepticism and the Ivermectin Debate
Ivermectin has not been without controversy in recent years. Its promotion by some as a potential antiviral during the COVID-19 pandemic sparked heated debates. Although many of those claims did not stand up to rigorous scientific validation, the public interest in repurposing existing drugs soared. This history makes the current push for ivermectin in cancer treatment a subject that must be approached with balanced skepticism and rigorous scientific inquiry.
It is important to differentiate between anecdotal reports and well-established clinical evidence. The ongoing study is designed to navigate these tricky parts through carefully controlled dosing schedules, thorough monitoring, and well-defined endpoints. By maintaining a neutral and scientific outlook, researchers aim to uncover the truth behind ivermectin’s potential without falling prey to hype or misinterpretation.
Clinical Eligibility: Who Qualifies for This Experimental Treatment?
Given the innovative nature of the trial, not every patient with TNBC will be eligible to participate. The eligibility criteria are intentionally strict to ensure that participants are likely to benefit from the treatment without undue risk. The key requirements include:
- Age and Performance: Only adults aged 18 years or older with an ECOG performance status of 0-1 are considered. This means the patients need to be in relatively good physical condition.
- Histological Confirmation: Participants must have metastatic triple-negative breast cancer confirmed through tissue studies, with specific low expressions of estrogen and progesterone receptors as well as HER2 negativity.
- Prior Treatment Failure: Patients must have experienced progression after one or two previous systemic treatments in the metastatic setting, ensuring that the trial is reserved for those in desperate need of advanced therapies.
- Exclusion Criteria: Individuals who have undergone prior PD-1/PD-L1 inhibitor therapy for metastatic disease or who are battling active autoimmune diseases, uncontrolled infections, or dangerous central nervous system involvement are not eligible.
This detailed screening is critical in sorting out the small distinctions between patients who may respond favorably and those for whom the trial might pose more risks than benefits. By doing so, the study intends to provide a robust framework to analyze outcomes while protecting patient safety.
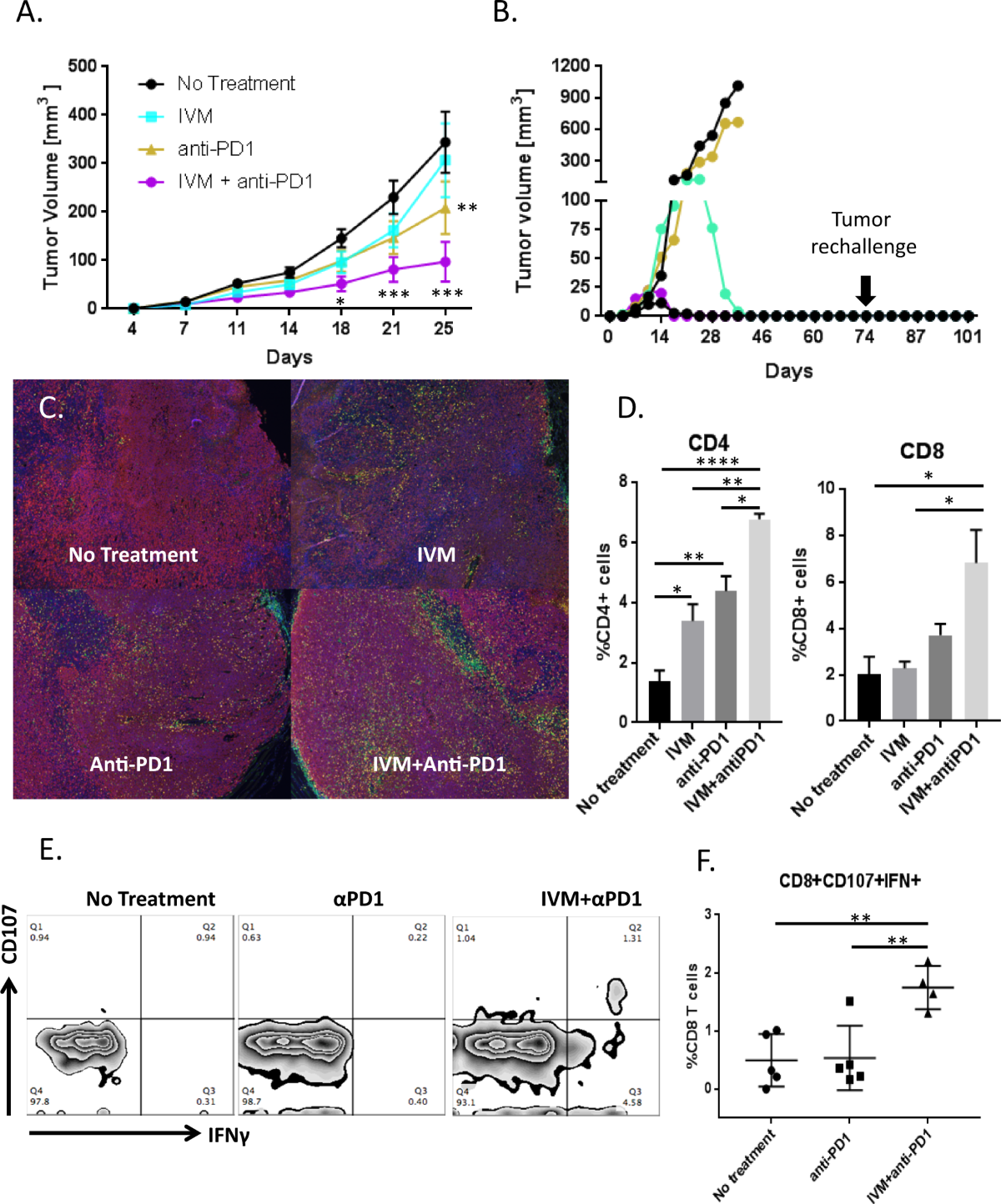
Synergy: How Ivermectin May Enhance Immunotherapy Efficacy
The combination of ivermectin with immune checkpoint inhibitors is rooted in the hypothesis that the drug’s ability to modify tumor pathways could complement the immune system’s natural cancer-fighting capabilities. Some of the vital aspects that researchers are investigating include:
- Shifting the Tumor Microenvironment: One of the challenges in cancer treatment is the tumor’s ability to create a protective shield, making it harder for the immune system to attack effectively. Ivermectin might help break down this shield.
- Reducing Cancer Stem-Like Cells: These cells are particularly resistant to treatment and can lead to recurrences. By lowering their numbers, the combination therapy could reduce the risk of relapse.
- Enhancing Immune Recognition: When tumor cells become more visible to immune cells, the overall effectiveness of immunotherapy can increase. Preclinical models have pointed to this possibility, although real-world data in patients remain to be thoroughly tested.
While each of these factors represents a small twist in the overall treatment strategy, together they form a compelling rationale for why combining ivermectin with established immunotherapies might yield better outcomes than either option on its own.
Challenges in Measuring Clinical Outcomes
One of the nerve-racking aspects of any advanced cancer study is measuring the real-world impact of a new treatment regimen. In the case of combining ivermectin with immunotherapy, several complicated pieces require careful oversight:
- Overall Survival Rates: Improvement in survival is an essential measure, but it can take considerable time to see statistically significant differences.
- Tumor Response Metrics: Imaging studies and biomarkers are used to evaluate whether the tumor is shrinking or becoming less aggressive. These assessments are complicated by the intermittent dosing schedule and the drug’s delayed effects.
- Quality of Life Assessments: Unlike drugs tested solely in the lab, clinical studies must also take into account how the treatment affects patients’ daily lives. This includes monitoring for side effects, both common and rare, and ensuring that improvements in tumor control translate to tangible benefits for patients.
In balancing these detailed measures, clinicians must figure a path through the overwhelming amount of data—from imaging studies to patient-reported outcomes—to reach a well-informed verdict on the treatment’s promise.
Comparing Ivermectin with Other Repurposed Medications in Oncology
The concept of repurposing drugs in cancer treatment is not entirely new. Other medications, originally intended for entirely different conditions, have been re-examined for their potential anticancer effects. Some prominent examples include:
- Metformin: Widely used in diabetes management, metformin has shown promise in intercepting cancer growth pathways.
- Statins: Primarily known for lowering cholesterol, statins have been investigated for their potential to reduce cancer cell proliferation.
- Aspirin: Beyond pain relief and cardiovascular protection, aspirin’s anti-inflammatory properties have led researchers to explore its role in reducing cancer risk.
These medications, like ivermectin, are attractive because they are already available, have a long history of safe use, and are relatively inexpensive. However, the key twist in every repurposing study is ensuring that the drug molecules interact with cancer cells in a meaningfully different way from their original indications. Ivermectin’s potential to both directly impact cancer cell survival and boost the immune system’s ability to recognize cancer cells makes it a particularly interesting candidate for further research.
Implications for Future Cancer Research and Treatment
If ongoing studies confirm that ivermectin enhances the effects of immunotherapy in conditions such as metastatic TNBC, the implications could be enormous. Not only would this provide a new option for patients facing an aggressive disease, but it might also pave the way for more research into repurposing other existing drugs for oncology. Some key implications include:
- Faster Access to New Therapies: Because drugs like ivermectin have already been used widely, the process of bringing them into the oncology fold could be quicker and less expensive than developing new drugs from scratch.
- Combination Treatment Models: The success of this study might help carve out a model for how traditional drugs can be paired with modern immunotherapies to create more robust treatment protocols.
- Improved Patient Outcomes: At the end of the day, the most important measure is whether patients can enjoy a longer, better quality of life with fewer side effects while fighting a disease known for its tricky parts and overwhelming challenges.
Such progress will likely prompt new research initiatives, increased trials globally, and a broader discussion on the role of drug repurposing in modern oncology. While there are still many small distinctions and subtle details to work out, the potential rewards are too significant to ignore.
Economic and Global Health Considerations
The economic side of repurposing drugs like ivermectin cannot be overstated. In many regions of the world, access to the latest, state-of-the-art cancer therapies is restricted by cost or availability. Ivermectin’s widespread use in other areas of global health indicates that it is both affordable and accessible. By adding another layer of utility to this common drug, health systems might be able to offer improved cancer care without the daunting price tag associated with many of today’s specialized cancer medications.
From an economic perspective, using a drug that is already mass-produced and distributed on a global scale could lower the overall cost of treatment. Often, the barrier to innovative cancer care isn’t just scientific but also fiscal. If a drug like ivermectin can be integrated quickly into existing cancer treatment protocols worldwide, it promises not only medical benefits but also broad-based public health improvements, especially in resource-limited settings.
Paths Forward: What Should Researchers and Clinicians Consider?
In wrapping up this exploration of ivermectin’s repurposing for cancer therapy, several key areas emerge as super important for future work. Researchers and clinicians need to:
- Conduct Larger, Controlled Trials: While early results are promising, larger studies will be needed to validate the benefits observed in smaller cohorts.
- Monitor Long-Term Outcomes: It is essential to track not just short-term tumor shrinkage, but also overall survival and quality of life over extended periods.
- Collaborate Across Borders: International collaboration could help pool data from diverse populations, ensuring that the treatment’s efficacy and safety are confirmed in a range of real-world settings.
- Address Patient Concerns: Given the prior public debate over ivermectin, transparent communication is needed to distinguish robust scientific evidence from anecdotal reports or politicized narratives.
By focusing on these areas, the medical community can work through the tangled issues and fine points of this promising strategy. Ultimately, it’s about offering patients improved treatment options in the face of cancers known for their overwhelming clinical challenges.
Final Thoughts: A Cautious Optimism for the Future
The journey from an old antiparasitic drug to a potential cancer treatment is filled with tricky parts and the ever-present risk of unexpected side effects. While the initial laboratory findings and early clinical trial designs are both promising and encouraging, it is important to maintain a balanced view. There is a need to acknowledge that the road ahead is riddled with tension and complicated pieces that must be understood through careful, diligent research.
At the same time, the prospect of enhancing immunotherapy – a field that has already revolutionized cancer care – with a familiar, widely available drug like ivermectin is super important. For many patients facing aggressive cancers such as metastatic triple-negative breast cancer, even incremental improvements in survival or quality of life can make a huge difference.
Weighing the Evidence: The Role of Scientific Rigor and Patient Advocacy
As this research unfolds, it is crucial for the medical community to dig into the fine details, ensure rigorous data collection, and openly share both successes and failures. Patient advocacy groups, clinical researchers, and regulatory bodies all have a role in ensuring that innovative treatments are both safe and effective. In the current climate, where misinformation can sometimes overshadow sound science, maintaining transparent communication with patients is key.
Both positive outcomes and potential setbacks should be discussed openly. By managing your way through the challenges – from dosing uncertainties to tracking long-term survival – researchers can build a more complete picture of how well ivermectin works in combination with immunotherapy.
What This Means for Current and Future Cancer Care
If the trials confirm that ivermectin can indeed enhance the effects of immunotherapy, this could reshape treatment protocols not only for TNBC but potentially for other cancers as well. Here’s a quick look at what this success might mean for the broader field:
- Expanded Treatment Options: Doctors could have a new tool at their disposal for treating aggressive cancers, providing hope for patients with limited alternatives.
- Reduced Treatment Costs: Repurposed drugs carry a lower price tag compared to novel, brand-new medications, potentially easing the financial burden on healthcare systems and patients alike.
- Accelerated Global Adoption: Given ivermectin’s existing distribution network worldwide, successful repurposing could lead to rapid integration into cancer care, even in lower-resource settings.
- New Research Avenues: Success in this area will likely spark further investigations into other repurposed medications, creating additional opportunities for breakthroughs in cancer treatment.
This potential shift underscores how flexible and innovative approaches can help us figure a path through even the most tangled issues in modern oncology. With patient needs at the forefront, such research efforts could fundamentally change how we approach cancer treatment in the years to come.
Reaching a Conclusion: Balancing Hopes with Real-World Data
Every new approach in medicine starts with a spark of hope, followed by rigorous testing under the microscope of scientific study. The journey of ivermectin from an antiparasitic marvel to a potential partner in cancer therapy illustrates the unpredictable, mixed journey of medical innovation. While we are excited by the possibility, there is also a need to remain cautious and evidence-based.
Currently, the ongoing trial serves as a critical platform for exploring several small distinctions in drug behavior and patient response. The combination of ivermectin with immunotherapy takes advantage of the drug’s known safety profile while testing its limits in a new and challenging environment. The data collected will help us steer through the confusing bits and reveal whether this strategy can be successfully translated into clinical practice.
The Road Ahead: Looking Beyond Initial Findings
As researchers continue to gather data from the trial, the oncology community – along with patients and caregivers – will be watching closely. Here are some additional considerations and steps for the future:
- Ongoing Data Collection: Long-term follow-up studies will be essential to understand the durability of the treatment response, especially in a disease with as many twists and turns as TNBC.
- Broader Application Studies: If initial results are positive, subsequent trials may explore the application of this combination in other types of cancer, potentially offering benefits across a wider patient spectrum.
- Patient Quality of Life: In addition to traditional endpoints like survival and tumor response, future research must pay close attention to quality-of-life metrics, which often capture the true impact of treatment on daily living.
- Collaborative Research Networks: By working together and sharing data internationally, researchers can overcome many of the confusing bits and hidden complexities associated with early-phase clinical trials.
While the current findings form an encouraging base, the trial’s results will ultimately determine whether ivermectin can carve out a lasting role in cancer therapy. Until then, the perspective remains one of cautious optimism – a balance of hope and scientific rigor.
Final Reflections: An Opportunity for Transformation in Oncology
The repurposing of ivermectin for cancer treatment highlights a broader trend in modern oncology: the search for innovative solutions by rethinking what is already available. In a field often loaded with off-putting financial and clinical barriers, this approach offers a refreshing possibility to maximize the potential of well-known medications.
For patients facing aggressive cancers, even small improvements in treatment can lead to super important benefits – both in extended survival and improved quality of life. Clinicians, researchers, and policy-makers must all work together to figure a path through the nerve-racking challenges and subtle details of these advanced therapies.
In Conclusion
The exploration of ivermectin’s role alongside immunotherapy represents a bold step in modern oncology. Though there are many obstacles – from ensuring patient safety to measuring long-term outcomes – the possibility of enhancing traditional immunotherapy with an affordable, widely available drug is undeniably attractive.
As the clinical data continues to accumulate, the medical community will have more clues to help them steer through both the promising and the perplexing pieces of this research. For now, the trial at Cedars-Sinai Medical Center stands as a beacon of hope and a testament to the innovative spirit that has always defined cancer research.
Ultimately, whether ivermectin evolves into a widely accepted component of cancer care or serves as a stepping stone for further research, its journey underscores the importance of remaining open to new ideas, even when those ideas come from unexpected corners of the pharmacological world. In these challenging times, when every small improvement matters, it is our collective responsibility to support research that could one day transform the landscape of oncology.
Originally Post From https://oncodaily.com/oncolibrary/ivermectin-and-immunotherapy
Read more about this topic at
Ivermectin converts cold tumors hot and synergizes with …
Ivermectin, a potential anticancer drug derived from an …