Emerging Developments in Cancer Treatment: A Closer Look at Recent Clinical Trial Data
The world of oncology is continually evolving with novel approaches intended to offer hope and improved quality of life for patients with life-threatening diseases. Recent reports from Karyopharm Therapeutics offer a window into how new cancer treatments, specifically selinexor monotherapy, are showing promise in the battle against challenging conditions like myelofibrosis. This opinion editorial takes a closer look into the clinical data, the delicate balance of efficacy and safety, and the broader implications for modern healthcare.
Understanding the Study and Its Context
In light of the press release from Karyopharm Therapeutics, the Phase 2 XPORT-MF-035 trial has sparked considerable attention in the oncology community. The trial focused on patients with hard-to-treat, heavily pretreated myelofibrosis – a population that has often been left with limited treatment options. In presenting this data at the 2025 European Hematology Association Annual Meeting, Karyopharm not only highlights the progress in drug development but also opens the door to many questions regarding the delicate balance of risk and reward in cancer therapy.
The data, which includes measurements of spleen volume reduction, hemoglobin stability, and reduction in inflammatory cytokines, represents steps forward in managing this challenging disease process. For those who face the overwhelming twists and turns of cancer treatment decision-making, these results offer a mixed bag of optimism tempered with cautious realism.
Key Clinical Insights and What They Mean for Patients
Clinical Activity in Hard-to-Treat Myelofibrosis
The trial’s findings indicate that selinexor monotherapy produced a spleen volume reduction of 25% or more in 67% of efficacy-evaluable patients treated with the drug. When placed side-by-side with physician’s choice (PC) treatments, the difference in outcomes, although promising, raises important points for consideration:
- Spleen Volume Reduction (SVR): The statistically meaningful reduction in spleen size is crucial because an enlarged spleen often contributes to painful symptoms and can compromise quality of life.
- Hemoglobin Stabilization: Maintaining hemoglobin levels is another key achievement, as lowering the need for frequent red blood cell transfusions greatly affects daily living.
- Cytokine Profile Improvement: Reductions in cytokines like IL-6, IL-8, TNFα, and hepcidin suggest that selinexor can moderate the inflammatory milieu — a problematic aspect loaded with issues in myelofibrosis pathogenesis.
Such details are crucial when considering treatment options for patients with a history of extensive therapy failures. It is important to note that these results provide hope even as many questions remain regarding long-term benefits and side effects.
Risks, Safety, and the Balancing Act of Novel Therapies
Assessing the Adverse Effects: A Balancing Act
Every revolutionary cancer treatment faces the challenge of balancing benefit with potential risk. In this trial, the most common treatment-emergent adverse events (TEAEs) were weight loss, nausea, anemia, and thrombocytopenia. It is essential for physicians and patients alike to understand these side effects—even though the study reported no treatment discontinuations due to TEAEs in the selinexor arm—so they can work through the tricky parts and manage care proactively.
Adverse reactions in such studies often remind us that every new approach has its share of overwhelming and sometimes nerve-racking challenges. The following table summarizes the key safety findings from the study:
| Adverse Event | Selinexor (%) | Physician’s Choice (%) |
|---|---|---|
| Decreased Weight | 50 | 50 |
| Anemia | 25 | 58 |
| Nausea | 33 | 25 |
| Thrombocytopenia | 33 | 25 |
| Upper Respiratory Tract Infection | 25 | 33 |
Items such as decreased appetite and cardiac failure were also noted as Grade 3+ TEAEs in some participants. These adverse events should be taken into account when considering treatment options, as they represent the fine points of drug tolerance and effect on quality of life. The data reinforces the age-old lesson that significant improvements in health outcomes are sometimes coupled with complex and nerve-racking risks.
Exploring the Broader Implications of Selinexor in Oncology
Shifting Paradigms in Cancer Treatment
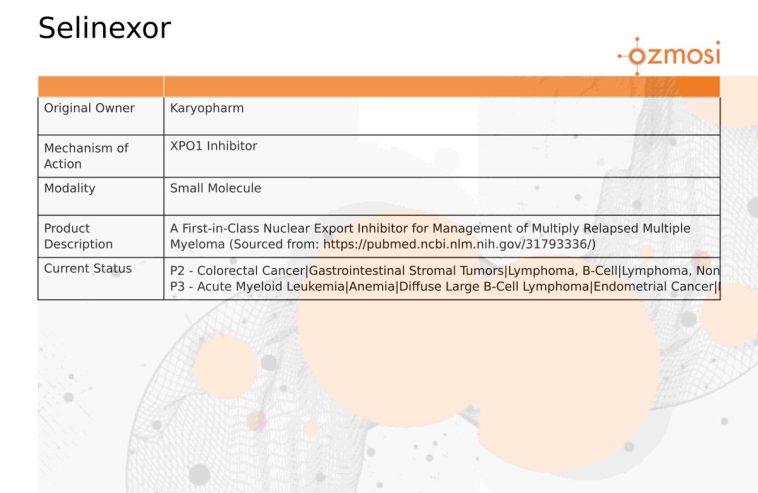
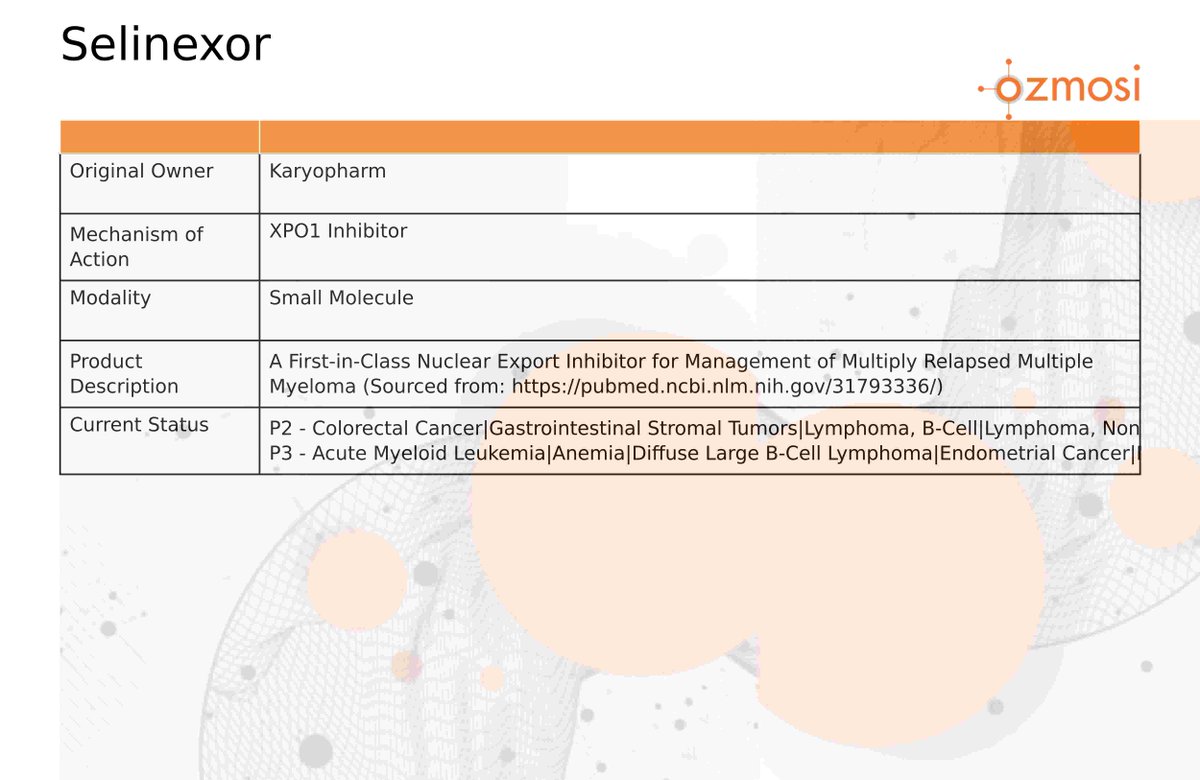
The introduction of selinexor (commercially known as XPOVIO® or, in some countries, NEXPOVIO®) presents a new way of treating cancers that have so far been resistant to standard therapies. Selinexor’s mechanism of action—selectively binding to and inhibiting the nuclear export protein XPO1—marks a shift from conventional treatments to a targeted approach that addresses the very underpinnings of oncogenesis.
This shift signifies more than just one drug’s journey; it reflects a broader move in oncology towards therapies that are both innovative and built upon nuanced understandings of cellular mechanisms. By working through the little details of nuclear export, researchers are getting into the heart of what makes some cancers so resistant to treatment.
But as with all pioneering techniques, there are twists and turns. The off-putting reality is that while early clinical data may be promising, long-term success, including durability of response and minimization of adverse effects, remains on the horizon. In essence, the fine points of this therapy’s effects still require further exploration over extended clinical trials.
Integrating Traditional and Alternative Theories in Cancer Management
Bridging Conventional Care with Innovative Approaches
While the primary focus of the report has been on an innovative pharmaceutical approach, it also invites us to consider the potential intersection between modern medicine and alternative approaches to cancer care. In many cases, patients find themselves juggling between standard therapies and complementary treatments, seeking a way to manage the confusing bits of side effects, improve overall well-being, and enhance quality of life.
Here are some of the ways that innovative cancer treatments might integrate with complementary approaches:
- Nutritional Interventions: Diet and nutrition form a cornerstone in managing side effects, improving energy levels, and supporting overall immune function during cancer therapy.
- Holistic Support: Techniques such as mindfulness, yoga, and meditation can help patients manage the overwhelming fears and anxiety often associated with a cancer diagnosis.
- Alternative Medicine: Acupuncture and herbal supplements are sometimes explored to alleviate pain and assist with nausea, although these should only be undertaken after thorough discussion with healthcare providers.
Combining the strengths of targeted pharmaceutical agents like selinexor with complementary therapies can create a comprehensive strategy that is both innovative and patient-centered. While the pharmaceutical data provides a critical piece of the puzzle, blending it with supportive, alternative measures may help patients find a more balanced approach in managing their health.
Assessing Drug Efficacy: The Evidence Behind the Data
Dissecting the Study Metrics
A closer look at the metrics from the XPORT-MF-035 trial reveals a narrative full of encouraging signals but also emphasizes that every drug must be carefully scrutinized on every level. The key clinical endpoints included:
- Spleen Volume Reduction (SVR25 and SVR35): These measures indicate that a significant number of patients experienced a reduction in spleen size. For patients with myelofibrosis, this reduction can translate to a decrease in abdominal discomfort and improved overall functionality.
- Hemoglobin Stabilization and Transfusion Rates: Higher mean hemoglobin levels and reduced red blood cell transfusion requirements are super important, as these endpoints directly impact patient energy and quality of life.
- Cytokine Dynamics: The differences in cytokine levels between the selinexor-treated and PC-treated groups offer insights into how the therapy might change disease activity on a molecular level.
While these endpoints are promising, it is essential to dive in deeper into the subtle parts of the clinical trial process. The inclusion of an optional crossover for patients treated with physician’s choice, for instance, provides a unique opportunity to gauge selinexor’s benefits in a broader sense. Such design choices are critical when trying to find your way through a landscape of treatment options where every decision counts.
Regulatory Perspectives and the Pace of Innovation
How Fast Should Innovation Move in Cancer Therapy?
The regulatory environment for pharmaceuticals, especially in oncology, is both a protective barrier and a facilitator of rapid innovation. The accelerated approval of drugs like XPOVIO in multiple indications is a testament to the balancing act regulators maintain between allowing earlier access to promising drugs and ensuring that comprehensive safety data is collected.
The pace at which regulators review and permit innovative therapies can sometimes feel overwhelming or even nerve-racking to both providers and patients. However, the careful monitoring of adverse events and the detailed listing of contraindications in the approved labeling help ensure that even when new medicines hit the market quickly, safety remains paramount.
Moreover, the reporting requirements and the regular updates required by agencies such as the FDA and their international counterparts serve as a reminder that progress in drug development is as much about managing the little twists as it is about celebrating significant breakthroughs. This scenario underscores the importance of not only embracing new treatments but also keeping an eye on the sandpaper details of safety monitoring and post-market surveillance.
Navigating the Fine Points of Oncological Research Reporting
Beyond the Headlines: What the Data Really Tells Us
Press releases like the one from Karyopharm are designed to highlight key achievements and generate enthusiasm. However, it is critical for readers and healthcare professionals to dig into the details to fully appreciate the context and possible limitations of any study. Some of the factors that one must consider include:
- Sample Size and Statistical Power: The trial involved a relatively small cohort, and while the results are promising, further studies involving larger populations are necessary to confirm these findings.
- Comparative Efficacy: The comparison to physician’s choice therapy, despite showing a difference, must be interpreted with caution, as the exact nature of those alternative treatments may vary significantly from one region to another.
- Long-Term Outcomes: Current data reflects early clinical response; however, the fine shades of long-term survival, quality of life, and disease progression require additional research and extended follow-up periods.
By taking the time to poke around in the data and reading between the lines, oncologists and patients alike can ensure that they are equipped with a complete picture before making treatment decisions. This careful reading of the nitty-gritty details is critical in a field where every decision can have far-reaching consequences.
Implications for Clinical Practice and Patient Care
Clinical Decision Making in a Changing Landscape
For clinicians, the emergence of a new drug like selinexor brings both hope and multiple points of consideration. In managing patients who are already grappling with the overwhelming challenges of myelofibrosis, oncology practitioners must incorporate new clinical data into a comprehensive care strategy. This involves:
- Risk-Benefit Analysis: Weighing the potential benefits of reduced spleen size, improved hemoglobin levels, and modulatory effects on cytokines against the possible risks of adverse events.
- Patient-Centered Communication: Explaining the subtle details of clinical trial data in a manner that is both comprehensive and compassionate. This requires discussing the likely benefits, the possibility of side effects, and the importance of regular monitoring.
- Integrative Care Models: Encouraging patients to consider supportive measures such as nutritional counseling, stress management, and, where appropriate, complementary therapies that might make the journey through treatment a bit more manageable.
By integrating these elements into a coherent treatment plan, healthcare providers can help patients find their path through what is often a tangled web of decisions. The ability to steer through such decisions effectively often determines the overall success of the treatment regimen.
Looking Ahead: The Future of Targeted Cancer Therapies
Innovating at the Intersection of Science and Compassion
The story of selinexor represents a broader trend in oncology: the ongoing quest to identify, understand, and target the underlying mechanisms that drive cancer. While these novel strategies can appear intimidating at first glance, they also offer a more precise alternative to conventional therapies that have long been the mainstay of cancer treatment.
Looking to the future, several key areas are expected to shape the oncological landscape:
- Personalized Medicine: As more data becomes available on the molecular profiles of individual tumors, there is potential to tailor therapies to meet the specific needs of each patient.
- Combination Therapies: While selinexor has shown promise as a monotherapy, there is also significant interest in exploring its use in combination with other drugs. These combinations might help mitigate side effects while enhancing overall efficacy.
- Continuous Monitoring: New methods for monitoring patient responses and potential toxicities through biomarkers and imaging techniques are emerging, which will play a key role in refining treatment protocols.
Every step forward in targeted therapy offers not only a means to combat the disease but also a way to understand it more deeply. By diving in to these scientific challenges and maintaining an open dialogue between researchers, clinicians, and patients, the future of cancer care looks both innovative and promising.
Understanding the Economic and Healthcare System Challenges
Cost Implications and Access to New Therapies
While the scientific puzzle of improved cancer treatment continues to be solved, the economic aspects also deserve attention. New drugs such as selinexor often come with high price tags, which can be intimidating and off-putting for patients and healthcare systems alike. It is essential to factor in:
- Cost-Effectiveness: The importance of balancing the cost of innovative therapy with the potential benefits in terms of survival and improved quality of life.
- Insurance Coverage: How insurance companies and governmental health programs address these new treatment modalities can deeply affect accessibility, especially in under-resourced communities.
- Long-Term Economic Benefits: Reductions in hospital admissions, fewer transfusions, and improved patient outcomes may ultimately contribute to lower overall healthcare costs, even if the upfront expense of the drug is high.
These factors remind us that while clinical innovation is paramount, the actual implementation of new therapies requires coordinated efforts from industry, regulators, and insurers. Only by finding your way through these economic and logistic challenges can the true benefits of these innovations be realized on a wider scale.
Patient Perspectives: Living With New Treatment Options
The Impact of Novel Therapies on Daily Life
From a patient’s point of view, the introduction of a new therapy like selinexor can be both inspiring and nerve-racking. Many patients view these advancements as a beacon of hope, especially when faced with diseases that are loaded with issues and have previously offered few alternatives. However, the path to improved outcomes is often paved with multiple twists and turns.
Patients must contend with not only the physical symptoms of their disease but also the emotional and psychological toll of embarking on a new treatment regimen. The journey involves:
- Managing Side Effects: While adverse events may be manageable with proper care, the uncertainty of new side effects requires constant close monitoring and patient education.
- Adjusting to New Routines: The transition from traditional therapies to novel agents often means changes in schedules, dietary restrictions, and additional visits to healthcare providers.
- Emotional Stress: The challenge of having to trust a new treatment, especially in cases where previous therapies have failed, can bring up feelings of vulnerability and anxiety.
Recognizing these human aspects of receiving a new treatment is super important. By ensuring that patients are well-informed about the benefits, potential risks, and the required monitoring, healthcare providers can help lighten the load for those navigating these nerve-racking changes in their treatment plan.
Real-World Applications and the Role of Healthcare Professionals
Integrating Clinical Trial Data into Everyday Practice
One of the main tasks for healthcare professionals is to translate promising clinical trial data into everyday practice. The evolution of cancer care is never a straight path, and every new piece of data must be carefully intertwined with existing treatment algorithms. For physicians, this means:
- Reviewing the Data Thoroughly: It’s essential to sort out the clinical efficacy, safety findings, and potential benefits for the individual patient. This includes understanding both the small distinctions in the study results and the broader implications for treatment strategies.
- Educating Patients: Medical professionals need to spend time explaining the fine details of how novel therapies like selinexor function and what patients can expect. Simplifying these explanations without oversimplifying the science is a key challenge.
- Ongoing Monitoring: As new therapies are introduced into routine practice, the monitoring for adverse effects and long-term outcomes becomes super important. This ensures that any issues can be addressed promptly and adjustments can be made as necessary.
The collaborative dialogue between physicians, nurses, and allied health professionals helps ensure that the benefits seen in a controlled clinical trial setting are realized in the day-to-day care of patients, offering a roadmap for managing the mixed results that arise with every new treatment.
Scientific Rigor and the Need for Continued Innovation
Progress Amidst the Challenges
The clinical trial data presented by Karyopharm rekindles the conversation about how difficult—and yet how crucial—it is to keep pushing the envelope in cancer research. Scientific breakthroughs rarely occur without a significant exchange of ideas, careful study, and lots of feedback loops. The journey from a promising hypothesis to a fully approved treatment is often full of complicated pieces that require continuous refinement. Key reflections include:
- Iterative Improvement: Each clinical trial adds another layer of understanding, helping researchers identify which aspects of a therapy work well and which need further adjustment.
- Real-World Evidence Gathering: Post-approval studies and observational data in the real-world setting are needed to verify the positive trends seen in early trials and to catch any confusing bits that might not have been evident in the shorter study period.
- Global Collaboration: The importance of pooling knowledge from different regions helps to maximize the chances of finding a successful treatment approach that works for a wide range of patients.
These insights not only build confidence in the incremental progress of cancer therapy but also encourage further innovative designs that might better address the tough challenges presented by resistant or aggressive cancers.
Balancing Innovation with Patient Safety Concerns
Ensuring Patient Welfare in the Era of New Therapies
Advances in cancer treatment come with the undeniable obligation to balance innovation with patient safety. Even as new treatments offer promise, the need to manage the potential for adverse effects is critical. The rigorous safety protocols and detailed adverse event guidelines associated with selinexor are an example of this balancing act. Key aspects for ensuring patient welfare include:
- Regular Monitoring of Blood Counts: Given the risk of anemia, thrombocytopenia, and neutropenia, clinicians must carefully track blood parameters throughout the course of therapy.
- Proactive Management of Side Effects: The implementation of supportive care measures – including antiemetics for nausea, appetite stimulants for decreased consumption, and transfusions when necessary – ensures that patients can continue therapy without undue discomfort.
- Patient Education on Red Flags: Educating patients on the subtle parts of their symptoms, such as signs of infection or cardiovascular concerns, empowers them to get prompt medical care when needed.
This delicate interplay of discovery and vigilance is a reminder to both patients and providers: while every scientific breakthrough holds potential, it must be continually evaluated against the real-world backdrop of patient health and safety.
Embracing the Future: The Role of Ongoing Research and Collaboration
Collaborative Efforts in Advancing Cancer Therapy
As the data surrounding selinexor continues to accumulate, the role of ongoing research grows ever more critical. Clinical trials like XPORT-MF-035 lay the groundwork for future investigations, generating hypotheses that may pave the way for even more refined therapies. Key areas where collaboration is essential include:
- Interdisciplinary Study Teams: Bringing together oncologists, hematologists, pharmacologists, and even experts in nutrition and alternative medicine fosters a well-rounded understanding of both the benefits and challenges associated with new treatments.
- Patient Advocacy: Involving patients and advocacy groups in research design and discussion helps ensure that the therapies developed meet real-world needs and address the everyday challenges faced by those living with cancer.
- Global Data Sharing: International collaborations and data-sharing initiatives allow for a more robust evaluation of emerging therapies and can lead to more rapid improvements in treatment approaches.
These cooperative efforts represent a commitment not just to innovation, but to reshaping the way we approach treatment in a holistic, patient-sensitive manner. This level of collaboration is key to ensuring that progress in oncology is both scientific and compassionate.
Conclusion: A Promising Yet Cautious Outlook on Cancer Therapy
Weighing the Pros and Cons of New Developments
The ongoing journey to combat cancer is a testament to the spirit of innovation and the desire to improve patient outcomes. Reports of selinexor’s promising results in heavily pretreated myelofibrosis patients bring renewed hope to patients and clinicians alike. While the preliminary data provide a reason to be optimistic, the path forward remains filled with both promising breakthroughs and the nerve-racking challenges of managing serious adverse events.
In the realm of oncology, every new treatment must be considered from multiple angles: from the clinical benefits of reduced spleen volume and stabilized hemoglobin, to the more subtle yet critical challenges of managing side effects and ensuring long-term safety. For every advancement in targeted therapies, there are equally important responsibilities in patient counseling, meticulous monitoring, and cost management.
Ultimately, the story of selinexor and its impact on the management of myelofibrosis is not a tale of unbridled success or failure. Rather, it is a reflection of the ongoing, iterative process of innovation in modern medicine—a process that requires us to get into the fine points of science, work through the little details of patient care, and remain open to integrating supportive therapies.
As we continue to figure a path through the ever-changing landscape of cancer treatment, it is essential to remember that every breakthrough comes with its own set of challenges. In embracing these new therapies, the healthcare community must balance hope with caution, drive innovation while ensuring patient safety, and remain ever mindful of the human lives behind each statistic.
While selinexor’s journey is still unfolding, its early clinical success suggests that it may become a key piece in the puzzle of modern cancer care. The need to steer through both the promising outcomes and the inevitable side effects underscores the multifaceted nature of oncology—a field where even the smallest twist can make a significant difference in patient outcomes.
In closing, as healthcare professionals, researchers, and patients together navigate the evolving twists and turns of cancer treatment, this story stands as a reminder of the impressive strides being made in modern medicine. It is a call to continue pushing the boundaries of scientific inquiry, all while never losing sight of the critical, everyday needs of those we serve. The future of cancer therapy hinges on a delicate balance, one in which every side effect is closely managed, every benefit celebrated, and every new discovery embraced with both scientific rigor and compassionate care.
Originally Post From https://investors.karyopharm.com/2025-05-14-Karyopharm-Announces-Poster-Presentation-on-Selinexor-in-Myelofibrosis-at-the-2025-European-Hematology-Association-Annual-Meeting
Read more about this topic at
Karyopharm Announces Poster Presentation on Selinexor …
Karyopharm Reports First Quarter 2025 Financial Results …