Emerging Advancements in Extensive-Stage Small Cell Lung Cancer Treatment
The treatment landscape for extensive-stage small cell lung cancer (ES-SCLC) is rapidly evolving. As an editor at an online healthcare journal, I have been closely following the transitions from traditional chemotherapy to more innovative approaches, and it is inspiring to see how novel therapies are reshaping what was once seen as a nerve-racking situation for patients. In this editorial, I take a closer look at the recent FDA approval of tarlatamab-dlle (Imdelltra), a DLL3/CD3 T-cell engager, and what this means for patients with relapsed or refractory ES-SCLC who have already undergone frontline chemoimmunotherapy.
It’s important to note that the field of oncology is constantly coping with tricky parts and tangled issues that challenge both clinicians and researchers. The introduction of therapies like tarlatamab is not just about adding another drug—it represents a quantum leap in addressing the small but significant twists and turns of ES-SCLC treatment. In this piece, I will explore the standard treatment options, clinical trial outcomes, and expert opinions that have culminated in this new approach becoming the second-line treatment standard for relapsed ES-SCLC.
From Chemoimmunotherapy to Innovative T-Cell Engagers
Frontline therapy for ES-SCLC has evolved over the past decade. Initially dominated by chemotherapy, the integration of immunotherapy agents such as atezolizumab (Tecentriq) and durvalumab (Imfinzi) marked a turning point. These treatments, used in combination with chemotherapy, have provided patients with improved outcomes and a more hopeful prognosis. The standard-of-care today involves a chemoimmunotherapy combination followed by an immunotherapy maintenance strategy.
However, when patients become refractory or relapse after these first-line treatments, clinicians are left with limited and often intimidating options. Traditionally, topotecan was the only approved chemotherapy for second-line treatment, with lurbinectedin (Zepzelca) emerging more recently. Both options, while effective to a degree, often left patients and their caregivers coping with overwhelming side effects and a general sense of limited hope.
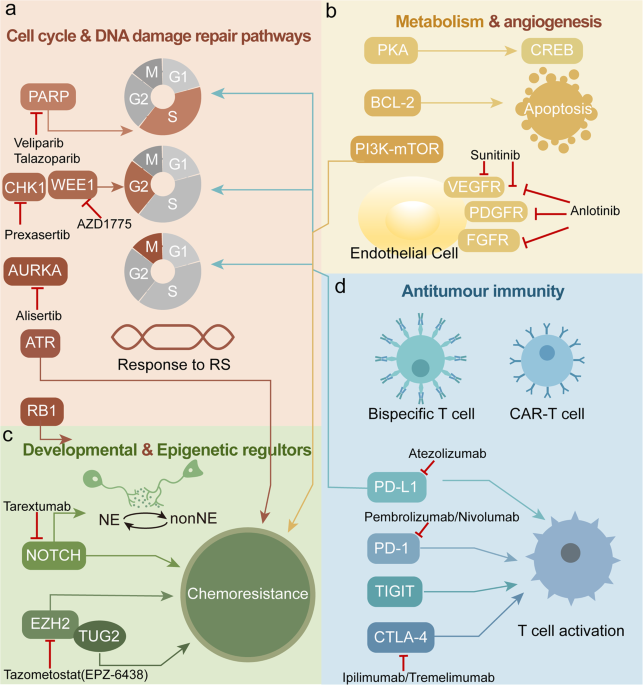
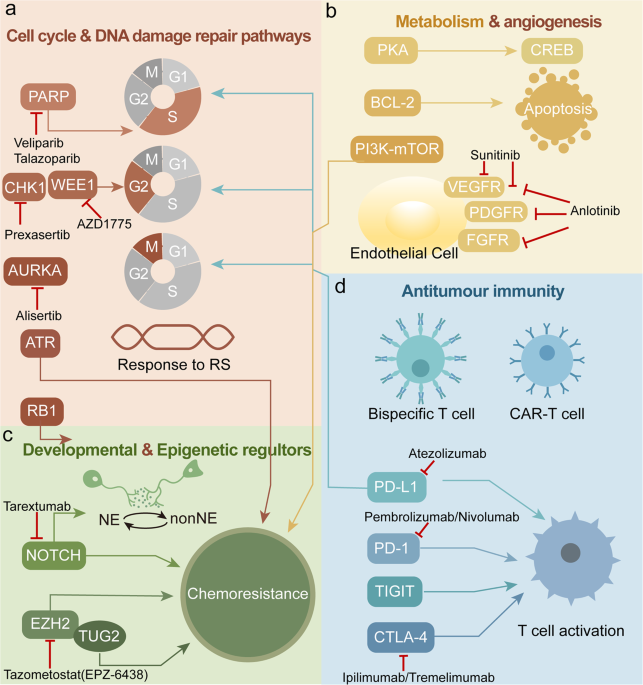
In contrast, tarlatamab represents an essential shift in the treatment paradigm—it is a T-cell engager that works by linking DLL3 on cancer cells with CD3 on T-cells, thereby mobilizing the body’s own immune system to target and destroy malignant cells. This shift is noteworthy not only from a clinical perspective but also in terms of patient quality of life. The idea of harnessing the immune system—an off-putting challenge in the past—is now becoming a super important component of cancer treatment strategies.
The Journey to FDA Approval: A Closer Look
Prior to its approval in May 2024, tarlatamab was undergoing rigorous clinical testing. The clinical trial data indicated that in patients with ES-SCLC whose disease had progressed on or after platinum-based chemotherapy, tarlatamab showed superior overall survival rates compared to existing standards of care. Such results are encouraging when one considers that around 70% of small cell lung cancer cases are diagnosed at the extensive-stage or with metastatic disease. For these patients, even small improvements in efficacy can make a significant difference.
Expert opinions have played a pivotal role in shaping this shift. Dr. Bingnan Zhang, an assistant professor at The University of Texas MD Anderson Cancer Center, provided insights into the evolving treatment options before and after the introduction of tarlatamab. In discussions with industry experts and through platforms like CancerNetwork®, Dr. Zhang highlighted that while chemotherapy remains central for most patients after frontline treatment failure, tarlatamab’s ability to engage T-cells represents a promising advancement that could eventually redefine the approach in second-line and beyond.
This journey to approval has not been straightforward. It involved a detailed assessment of trial design, survival analysis, and managing the confusing bits of immune-related adverse events. Researchers had to figure a path through a maze of complicated pieces and hidden complexities inherent in innovative cancer treatments. Such progress is not only an achievement in clinical oncology but also stands as a testament to the combined efforts of researchers, clinicians, and regulatory authorities.
Examining the Clinical Data Behind Tarlatamab
One of the key aspects of any new treatment approaching FDA approval is the underlying clinical data. When professionals poke around through the trial metrics for tarlatamab, several factors emerge as significant:
- Efficacy: Tarlatamab demonstrated improved overall survival and a better response rate compared to existing chemotherapy options. This is critical for patients who have already exhausted first-line options.
- Safety Profile: Although all new oncology drugs come with their own set of side effects, tarlatamab’s profile was found to be manageable. Experts noted that while there are risks, the balance between benefits and potential adverse effects tilts in favor of its use in heavily pretreated patients.
- Mechanism of Action: By harnessing the immune system through a DLL3/CD3 engagement, tarlatamab offers a targeted mechanism that may help overcome resistance seen with conventional chemotherapy.
A quick summary table comparing the various second-line treatment options for relapsed ES-SCLC might help clarify these points:
| Treatment Option | Mechanism | Response Rate | Common Side Effects |
|---|---|---|---|
| Topotecan | Chemotherapy | Moderate | Myelosuppression, nausea |
| Lurbinectedin | Chemotherapy | Moderate | Fatigue, hematologic toxicity |
| Tarlatamab | T-cell engagement (DLL3/CD3) | High (based on emerging data) | Immune-related events, manageable profile |
This table highlights some of the fine points and subtle differences between traditional chemotherapy agents and this innovative immunologic strategy. It is clear that while every treatment has its own set of challenges, tarlatamab offers a promising alternative that could alter the survival trajectory for many patients.
Real-World Impact: Patient Perspectives and Quality of Life
When we consider treatments purely from a clinical standpoint, it is easy to overlook what truly matters—the patients themselves. The introduction of tarlatamab is not just another scientific milestone: it has the potential to improve patient outcomes in ways that directly affect quality of life, daily functioning, and overall well-being.
Patients with ES-SCLC often face a nerve-racking journey filled with intimidating side effects and a limited number of effective treatment options. For many, the introduction of a treatment that not only targets the cancer more precisely but also carries a manageable side effect profile brings a breath of hope. By reducing reliance on harsh chemotherapies for subsequent lines of treatment, patients could experience fewer disruptive impacts on their everyday lives.
Many patients report feeling more empowered when presented with innovative treatment options. With tarlatamab, the possibility of a treatment that mobilizes the immune response adds a psychological boost, in addition to its clinical benefits. It provides patients an opportunity to actively engage with a treatment regimen that is tailored to overcome the tricky parts of cancer cell resistance, making it a must-have option in today’s treatment arsenal.
Expert Insights: Weighing the Benefits and Challenges
In any evolving treatment landscape, the opinions of seasoned professionals provide valuable insight. Dr. Zhang and other experts in the field have consistently emphasized that while the benefits of tarlatamab are promising, its integration into clinical practice must be done with both care and a clear understanding of the potential complications.
There are several factors to consider when embracing such advanced therapies:
- Managing the Immune Response: The engagement of T-cells is a powerful approach, but it comes with its own set of confusing bits, including immune-related adverse events. Properly managing these events requires a careful balance and an experienced healthcare team.
- Patient Selection: Not every patient with ES-SCLC may be an ideal candidate for tarlatamab. Determining who will benefit most from this therapy involves evaluating previous treatments, overall health, and the presence of organ metastases such as to the brain.
- Monitoring and Follow-Up: As with any novel treatment, close monitoring is key. Healthcare providers will need to keep a keen eye on patient responses, particularly in the early phases of treatment, to make necessary adjustments.
Experts agree that it is critical to sort out the details of patient selection early on. For patients who have already experienced the twists and turns of ES-SCLC and have faced a few too many overwhelming challenges with conventional chemotherapy, tarlatamab offers a new path forward. Still, as with every innovative approach, there are hidden complexities that require clear communication between patients and their healthcare teams.
Integrating Modern Medicine with a Personalized Approach
The approval of tarlatamab is part of a broader trend in oncology that focuses on personalized medicine. Rather than a one-size-fits-all approach, clinical strategies are increasingly tuned to the individual patient’s cancer profile, overall health status, and response history. This paradigm shift implies that while tarlatamab is a significant advancement for ES-SCLC, its success is amplified when integrated into a multidisciplinary treatment strategy.
Modern treatment centers are now embracing a combination of approaches—melding the latest in immunotherapy with supportive care measures that prioritize the patient’s overall quality of life. Personalized cancer care plans are built on a foundation of detailed molecular profiling, imaging studies, and in-depth patient history assessments. In this respect, the role of tarlatamab, with its targeted mechanism of action, is critical. It complements rather than replaces traditional treatment, forging a new way of tackling cancer that addresses both the disease and the patient’s specific needs.
Indeed, personalized care not only directs the use of advanced treatments but also helps mitigate potential side effects and minimize disruptions in daily life. Patients are increasingly becoming partners in decision-making as they work with their healthcare teams to figure a path through treatment options that best match their personal circumstances. Such collaborative efforts represent a key opportunity to improve both survival rates and quality of life.
The Broader Implications for Oncology Practice
The introduction of tarlatamab has far-reaching implications beyond just the treatment of ES-SCLC. It signifies a shift in how oncologists view the treatment puzzle in cancers that are loaded with problems and challenging treatment histories. By offering a mechanism that stimulates the body’s inherent immune response, tarlatamab provides a new tool in the oncologist’s arsenal—one that can potentially be adapted for other malignancies where conventional approaches have reached their limits.
In a broader sense, the approval of innovative therapies such as tarlatamab fuels optimism about the future of oncology. As researchers continue to dig into the nuances of cancer biology, additional immunotherapies and targeted agents are likely to emerge, further expanding the choices available to oncologists and their patients. This trend is not just an evolution in treatment—it is a revolution in how we understand and combat cancer on multiple fronts.
Furthermore, the ripple effects of such approval extend into healthcare policy, insurance coverage, and patient advocacy. With regulatory bodies recognizing the superiority of newer agents based on solid clinical evidence, there is a growing momentum to support research that pushes past the traditional boundaries of chemotherapy. This creates a wave of opportunity not only for pharmaceutical innovation but also for medical professionals and patients who are ready to embrace personalized and precision-based approaches.
Overcoming the Overwhelming Challenges: A Roadmap for the Future
The treatment of relapsed ES-SCLC has long been riddled with tension and complicated pieces. The introduction of tarlatamab offers a fresh perspective—a way to help steer through the maze of limited options that have long left patients feeling both overwhelmed and on edge. Nevertheless, its successful integration into clinical practice depends on several strategic factors:
- Robust Training and Education: Medical practitioners need continuous education and hands-on training to understand and manage the subtle details associated with novel immunotherapies.
- Interdisciplinary Collaboration: Oncologists, pharmacists, nurses, and supportive care teams must work closely to sort out the practical bits of dosing, monitoring, and adverse event management.
- Patient-Centric Protocols: It is super important that treatment plans place patient needs at the forefront. Side effects should be managed promptly to maintain patients’ quality of life while optimizing therapeutic outcomes.
- Ongoing Research and Feedback: As with any new treatment, collecting real-world data post-approval is critical. This continuous feedback loop will help refine the application of tarlatamab and inform future therapeutic strategies.
The above list is a practical roadmap that illustrates the necessary steps needed to integrate groundbreaking treatments while making sure the healthcare system adapts seamlessly to change. In overcoming the overwhelming challenge of relapsed ES-SCLC, every step, from clinical trials to real-world application, must be executed with precision and care.
Therapeutic Decision-Making: Balancing Efficacy and Patient Safety
At the heart of any treatment decision in oncology lies the need to balance the potential for improved survival with the risk of adverse effects. When comparing therapies like topotecan, lurbinectedin, and the new tarlatamab, several key considerations arise:
- Efficacy Outcomes: Improved response rates and overall survival are primary endpoints in clinical trials for advanced cancer therapies. The data supporting tarlatamab’s use in the relapsed setting indicate not only a superior response but also a valuable extension in quality-adjusted life years.
- Side Effect Profiles: While traditional chemotherapies carry a higher risk of myelosuppression and systemic toxicity, newer agents like tarlatamab provide a more targeted attack on cancer cells. This shift can translate into fewer complications such as nausea or severe fatigue, which are common with older treatments.
- Patient Demographics and Comorbidities: Every patient’s journey is unique. Comprehensive assessments—including age, performance status, and the presence of brain metastases—are critical in figuring a path towards the best individualized treatment choice.
These considerations make it clear that treatment decision-making in ES-SCLC is far more than a simple choice between medications. It is a detailed process that involves parsing through the little details, understanding the tricky parts of each drug’s mechanism, and aligning the treatment with the patient’s overall health goals.
Impact on the Treatment Algorithm: A Paradigm Shift
The approval of tarlatamab not only redefines the second-line treatment strategy for relapsed ES-SCLC but also has the potential to reshape existing treatment algorithms. Historically, the algorithm has been straightforward: administer frontline chemoimmunotherapy followed by second-line chemotherapy upon disease progression. However, with tarlatamab coming into the picture, oncologists are now granted an additional, more effective tool that could challenge the old order.
This paradigm shift can be visualized in the following simplified treatment algorithm:
- First-Line Therapy: Chemoimmunotherapy using platinum-based chemotherapy with maintenance PD-L1 inhibitors (atezolizumab or durvalumab).
- Second-Line and Beyond: Transition from conventional chemotherapies like topotecan and lurbinectedin to tarlatamab, offering patients a better overall survival outcome.
By expanding the treatment algorithm to include tarlatamab, the entire framework becomes more flexible and responsive to the evolving landscape of oncology. In the near future, we may see further modifications as additional therapies targeting related pathways are introduced. This flexibility is a super important quality, as it reflects the dynamic nature of modern medicine where innovation is constant.
Healthcare Policy, Access, and the Future of Oncology Innovation
Another critical dimension in this discussion is the role of healthcare policy and access. The introduction of any new treatment, particularly one that represents a significant shift from conventional methods, must be supported by a framework that ensures broad access and affordability for patients.
Policy makers and healthcare providers face several tasks in this arena:
- Insurance Coverage: Ensuring that treatments like tarlatamab are covered under standard oncology benefit plans so that patients are not left facing dire financial challenges.
- Funding for Research: Continued government and private funding for innovative therapies is essential to maintain the momentum seen in recent years.
- Clinical Infrastructure: As newer therapies become mainstream, clinical infrastructure must evolve to support the necessary training, monitoring, and management protocols required for safe administration.
The incorporation of novel treatments into national healthcare strategies is a super important tactic that enables comprehensive cancer care. In turn, this improves overall oncology outcomes and accelerates the adoption of practices that prioritize both efficacy and patient well-being.
Conclusion: Embracing the Future of ES-SCLC Therapy
In conclusion, the rise of tarlatamab-dlle as a new standard for treating relapsed ES-SCLC represents both a milestone and a harbinger for the future of oncology. Its approval underscores the importance of continuously stepping into new therapeutic territories—despite the intimidating and sometimes overwhelming challenges—to address critical needs in cancer treatment.
This editorial highlighted not only the promising clinical data and expert commentary surrounding tarlatamab but also its potential to reshape treatment algorithms and create a more patient-focused therapeutic landscape. By employing modern immunotherapy techniques, oncologists are now better equipped to find their way through the tangled issues of relapsed disease, ultimately offering renewed hope for patients who had once felt that their treatment options were limited.
As we look ahead, it is super important for continued collaboration among researchers, clinicians, policy makers, and patient advocates. Embracing these innovative therapies requires everyone to take the wheel and work together through the fine points of personalized medicine. In doing so, we pave the way for a future where even the trickiest parts of cancer treatment can evolve into clearer, more effective strategies that benefit everyone involved.
Ultimately, the FDA’s accelerated approval of tarlatamab serves as a reminder that the journey through cancer treatment is never-ending. With every new development—from chemoimmunotherapy to targeted T-cell engagement—we are better positioned to tackle the many twists and turns of the disease. It is a path filled with challenges, but also with promising opportunities that make modern cancer care not only more advanced but also more compassionate and patient-centered.
As modern medicine continues to push the boundaries of what is possible, we must remain committed to ensuring that these scientific advancements translate into tangible benefits for the patients who rely on them. Each step forward in treatment innovation is a step forward in our collective fight against cancer, and it reinforces the idea that with collaboration and perseverance, even the most intimidating obstacles can be overcome.
Let this new era of targeted immunotherapy be a beacon of hope—a sign that despite the overwhelming complexity and the nerve-racking challenges that cancer presents, medicine is continually evolving for the better. Together, by embracing both the science and the humanity behind patient care, we can build a future where no one faces a cancer diagnosis feeling isolated or without effective options.
Originally Post From https://www.cancernetwork.com/view/moving-beyond-chemotherapy-in-relapsed-refractory-es-sclc-treatment
Read more about this topic at
Introducing PROTAC therapy as a novel paradigm in …
Revolutionizing lung cancer treatment with smart …