Revolutionizing Early Cancer Detection: A New Frontier in Biomedical Engineering
The landscape of cancer research is undergoing a significant transformation, one that promises earlier detection, improved treatment, and ultimately, enhanced survival rates. In today’s discussion, we shine a light on the pioneering work emerging from institutions like Oregon Health & Science University (OHSU). Recent advancements in 3D bioprinting, organoids, and organs-on-a-chip are opening unprecedented avenues for researchers to study cancer’s onset. These innovative tools are not only enabling scientists to recreate the human body’s environment more accurately, they also offer fresh insights into the tricky parts of tumor initiation, making it possible to catch the disease before it develops into something far more intimidating.
Innovative Biofabrication for Personalized Cancer Diagnostics
One of the most influential aspects of modern cancer research has been the development of biofabrication techniques. These processes allow researchers to recreate healthy tissue and, with advanced tools, incrementally introduce changes that could lead to cancer. The traditional method of studying cancer has long depended on animal models, yet these systems often fall short when it comes to reflecting the real human cellular landscape. By contrast, new approach methodologies that use human-derived cells in lab-grown tissues offer a more relevant and precise insight into the early stages of cancer.
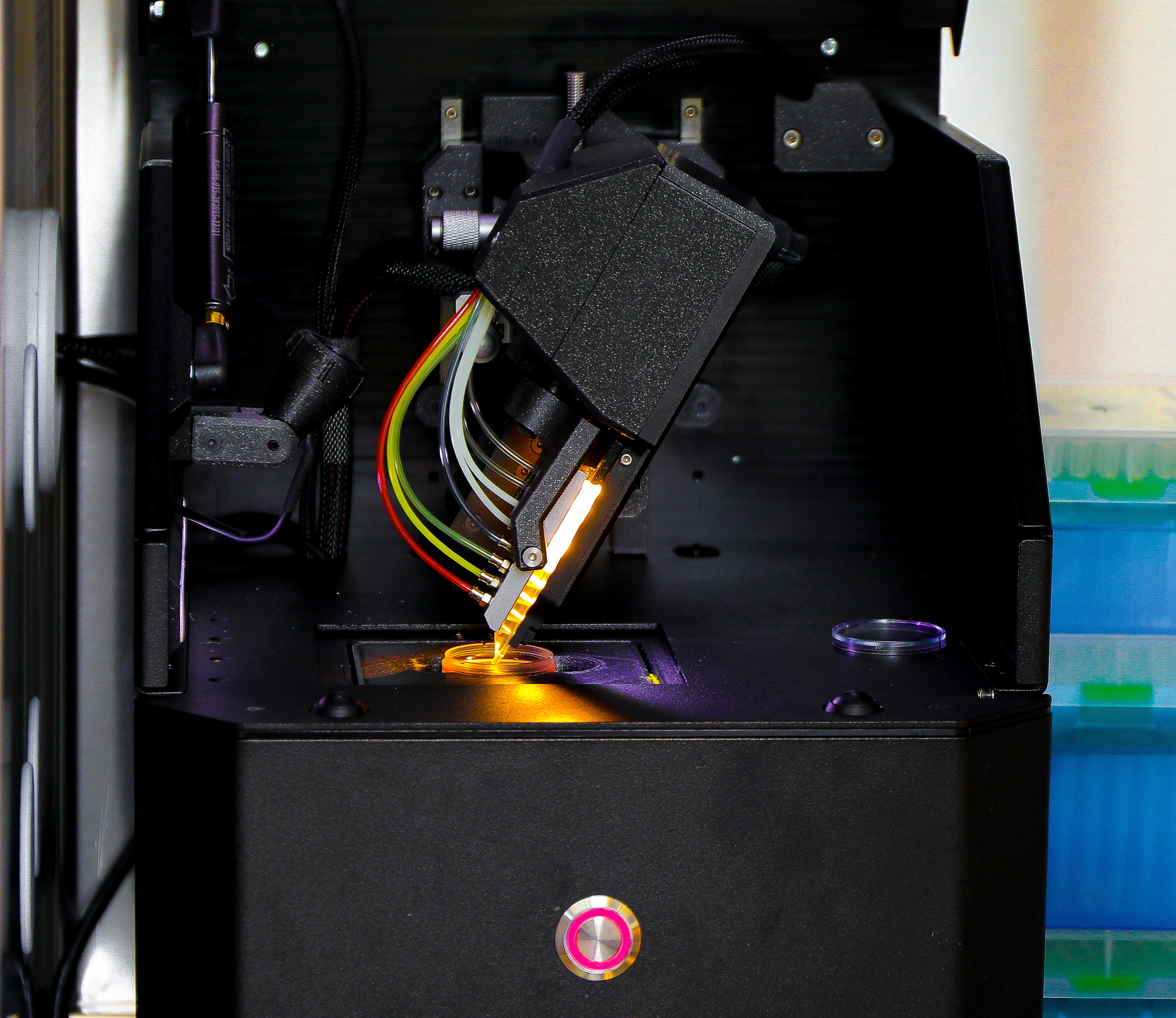
Using 3D bioprinting technology and microfluidic devices known as organs-on-a-chip, researchers can now build realistic tumor models. These models represent an environment that closely mimics the in vivo conditions, or what happens within the human body. By observing these lab-grown tissues, scientists are able to closely monitor the subtle parts of cancer evolution—from healthy cells gradually transforming into cancerous ones to the minute environmental changes that might trigger such a transformation.
This breakthrough in tissue engineering is crucial because it offers a window into the early twists and turns of cancer development. Getting into the early stages of cancer progression not only boosts survival rates by catching the disease sooner, but also sets the stage for personalized treatment strategies tailored to each patient’s unique genetic and cellular makeup.
Advanced Organoids and Organs-on-a-Chip: Capturing the Early Moments of Tumor Formation
Lab-generated models such as organoids and organs-on-a-chip are providing new ways to study cancer initiation. These systems are especially useful in mimicking the natural environment inside the human body, where multiple cell types communicate and interact with one another. Such a close simulation empowers researchers to poke around the early, and often confusing, bits of tissue transformation and to tease out the intricate details of how a tumor might emerge.
There are several benefits to these models:
- Realistic cellular interactions: They offer a genuinely human cell-based system where interactions are more true-to-life.
- Reduced reliance on animal testing: With heightened concerns about animal welfare and data applicability, these models pave the way for a transition to systems that mirror the human condition better.
- Precise manipulation: Scientists can adjust cellular and genetic factors to see exactly how specific conditions lead to tumor growth.
These tools are far from simply recreating the physical structure of tissues—they are designed to simulate the tangled issues and subtle details of the tumor microenvironment. This gives researchers the ability to observe how even slight environmental changes, such as alterations in nutrient supply or oxygen levels, can influence cancer behavior. With this enhanced understanding, clinicians may soon be able to diagnose early-stage cancer with greater certainty, making early treatments far more effective.
3D Bioprinting: Unraveling the Nitty-Gritty of Early Cancer Development
The evolution of 3D bioprinting has been a game changer in modern biomedical research. Nearly a decade after groundbreaking work in 3D printing blood vessels revolutionized the field, experts like Dr. Luiz Bertassoni are pushing the envelope even further. Using a sophisticated chip-based system that simulates the bone-tumor environment, his team is dissecting the fine points of cancer initiation that traditional models might overlook.
3D bioprinting offers several significant advantages:
| Advantage | Description |
|---|---|
| Precision Engineering | Enables the accurate recreation of a patient’s tumor environment, providing a detailed look at the early formation steps. |
| High Customizability | Allows for the creation of personalized tumor models that mirror the unique characteristics of individual patients’ cancers. |
| Scalability | Offers a platform to test a wide range of drugs and treatment approaches, making it easier to identify the most effective options. |
With these capabilities, scientists are steadily untangling the nerve-racking bits of early cancer transformation. They are able to observe not only the healthy-to-cancerous transition but also the impact of various cellular factors in an environment that closely resembles the real human body. This approach is vital for understanding potential cancer biomarkers—essential indicators or red flags that may help identify the disease in its nascent form.
Marrying Engineering, Biology, and Clinical Treatment: Opportunities and Challenges
When discussing early cancer detection, it is impossible to ignore the promising intersection of engineering, cancer biology, and clinical treatment. Despite the progress, there remain several tricky parts in this emerging field that researchers must address. One of the key obstacles is translating in vitro (lab-based) results to actionable clinical insights. While advanced models like organs-on-a-chip hold immense promise, their transition into routine clinical practices involves sorting out many complicated pieces and validating their effectiveness against established diagnostic methods.
Clinicians and scientists must work together to bridge the gap between lab-based discoveries and practical, everyday treatments. This synergy is critical, as it enables the design of screening tools that are not only technologically innovative but also patient-friendly and economically viable. With the increasing focus on “cancer interception” or intervening before a tumor fully forms, there is an essential opportunity to reframe cancer treatment from managing symptomatic disease to preventing its progression altogether.
This approach invites us all to think about cancer diagnosis in a new light. Instead of being overwhelmed by the intimidating variety of cancer types and their numerous pathways, we now have tools that let us figure a path through these complexities. Patient outcomes stand to benefit greatly if early detection can be paired with targeted, personalized therapies based on a deeper understanding of each tumor’s unique developmental trajectory.
State-of-the-Art Organoid Models: Understanding the Tumor Microenvironment
One of the most exciting developments in early cancer research is the use of organoids. Organoids are three-dimensional cell cultures that mirror the structure and functionality of real organs. These models are especially useful for studying the initial, subtle parts of cancer development in different types of tissues—from the breast and colon to the lung and liver.
This technology enables researchers to dive in and observe how cancers begin with a fine-tuned level of detail. In effect, organoids act as a bridge between two-dimensional cell cultures and real human tissues, providing a more realistic environment to test the hypothesis that certain cellular changes lead to cancer. By carefully controlling the culture conditions, scientists can trigger or suppress the early signs of malignancy and then study the little twists that indicate why cancer sometimes progresses from a small lesion to a full-blown tumor.
Some key benefits of organoid models are:
- Enhanced Biological Relevance: They better reflect the in vivo cell-to-cell interactions and microenvironment found in the human body.
- Controlled Environment: Researchers can manipulate factors like nutrient levels, which may have a direct impact on cellular behavior.
- High Predictive Power: These models are promising tools for predicting patient responses to specific therapies, laying the groundwork for personalized patient care.
Computational Modeling and Its Role in Early Cancer Detection
Alongside physical models, computational modeling is emerging as a pivotal component in early cancer detection. By simulating the behavior of cells and tissues on a computer, researchers can replicate the early stages of cancer before even stepping into the lab. This computational approach offers a unique advantage—it allows scientists to run simulations across a wide range of variables, testing many different scenarios in parallel.
Computational models help in piecing together several of the small distinctions involved in tumor initiation. Researchers can combine data from bioprinted tissues and organoid experiments to refine algorithms that predict cancer behavior. Ultimately, these methods could be used to develop diagnostic tools that forecast the onset of cancer well before it becomes visible through conventional imaging techniques.
A few notable benefits of applying computational modeling are:
- Time Efficiency: Simulations can be run much faster than physical experiments, accelerating the pace of discovery.
- Cost Effectiveness: They reduce the need for expensive and time-consuming laboratory tests.
- Holistic Analysis: These models integrate various data streams to give a comprehensive picture of the early tumor microenvironment.
When integrated with tangible, lab-based models, computer simulations help bridge the gap between theory and practice. The resulting insights empower clinicians to identify risk factors quickly, enabling earlier and more accurate diagnosis.
Real-World Impact: Translating Laboratory Advances to Clinical Practice
While scientific breakthroughs in 3D bioprinting and organoids are generating considerable excitement in academic circles, the bigger question remains: How soon can these innovations be translated into everyday clinical practice? Transitioning from a controlled lab setting to the real world presents several intimidating, yet solvable, challenges.
For these technologies to be adopted, researchers need to:
- Ensure Reproducibility: Any new method must reliably mimic the human system to be accepted in clinical settings.
- Validate Against Standard Diagnostics: It is crucial that these advanced models outperform or at least match the effectiveness of conventional techniques.
- Overcome Regulatory Hurdles: Moving from experimental phases to clinical applications requires navigating a nerve-racking regulatory process, one loaded with protocols designed to guarantee patient safety.
Despite these challenges, the promise of early detection and personalized treatment makes it an endeavor worth pursuing. With improved diagnostic accuracy, patients could face a future where cancers are intercepted before they progress to more complicated pieces, resulting in simpler and more effective treatments.
Integrating Multi-Disciplinary Research: A Collaborative Approach to Cancer Interception
The ongoing research at OHSU and similar institutions demonstrates the power of integrating diverse scientific expertise—from biomedical engineering and tissue engineering to computational modeling and clinical practice. This collaborative spirit is key to figuring a path through the tangled issues surrounding early cancer detection.
Working together, experts are not only building highly accurate tumor models in the lab but are also pushing to apply their discoveries in a way that directly benefits patients. A multi-disciplinary approach encourages innovative solutions where each discipline contributes its unique perspective to solving the whole problem. This cooperation is transforming early cancer detection from a concept into a clinic-ready reality.
In addition to technical innovations, this cooperation fuels hope. It promises an era where early detection through personalized biofabrication is not a niche interest confined to research labs but a standard part of cancer care, ensuring that patients receive the best possible intervention at a stage when treatment is most effective.
Merging Traditional Wisdom with Modern Technology
It is important to recognize that even as modern tools push the boundaries of cancer research, there is still enormous value in the traditional practices of medicine. Conventional diagnostic techniques, including imaging and laboratory tests, remain essential. However, integrating these established methods with advanced biofabrication and computational methods offers a more nuanced approach than ever before.
This merging of old and new allows for:
- Cross-Validation: Modern lab models can be aligned with historical data to improve overall diagnostic accuracy.
- Incremental Adoption: Clinicians can gradually incorporate new tools into their practice without an abrupt overhaul of established protocols.
- Enhanced Patient Care: A collaborative approach ensures that while technology refines detection, patient safety and care remain paramount.
Embracing both worlds is key to addressing the fine shades of early cancer development. Such a balanced approach underlines the necessity of not only developing novel diagnostic tools but also ensuring they are seamlessly incorporated into the existing healthcare framework.
Future Perspectives: What Lies Ahead for Early Cancer Diagnosis?
The rapid progression of technologies like 3D bioprinting and organs-on-a-chip suggests that we are on the brink of a new era in healthcare. As researchers continue to figure a path through the nerve-racking and tangled issues of early tumor detection, the future promises several key developments:
- Personalized Medicine Growth: With the advent of truly personalized tumor models, treatments could be tailored to each individual’s unique cellular profile, enhancing treatment outcomes and reducing side effects.
- Preventative Interventions: Early detection models pave the way for interventions that could stop cancer before it fully develops, shifting the focus from managing symptoms to proactive prevention.
- Better Risk Stratification: Utilizing a combination of computational and laboratory methods, clinicians may soon be able to classify patients by their specific risk profiles, ensuring that treatment urgency and protocols are perfectly matched to individual needs.
- Faster Drug Discovery: The advanced models also offer a platform to rapidly test new treatments, enabling a quicker turnaround from drug discovery to clinical trials.
Looking ahead, it appears that the integration of modern bioengineering with solid clinical practice will not only improve outcomes for patients but also fundamentally change our approach to battling cancer. As the field continues to grow, one thing is clear: the era of relying solely on conventional methods is gradually giving way to a more integrated, patient-centered future in cancer diagnostics and treatment.
Addressing the Challenges: Steering Through Regulatory and Ethical Considerations
Of course, this innovative field is not without its share of challenges. One of the most critical issues is navigating the regulatory systems that govern the use of new medical technologies. While there is widespread enthusiasm for these advancements, ensuring patient safety and proving clinical efficacy is of paramount importance. Regulatory agencies must now work with researchers to ensure that transitioning from the lab to clinical practice is done carefully and with full transparency.
Furthermore, ethical considerations are central to this evolution. As researchers and clinicians push forward with technologies that simulate human tissues and biology, it is vital to ensure that patients are fully informed and that consent is managed with the utmost respect for individual rights. Balancing the promise of future breakthroughs against the current, sometimes intimidating, regulatory frameworks is a fine line that everyone in the field must tread with care.
In addressing these challenges, several strategies are emerging:
- Collaborative Regulation: Bringing regulators, clinicians, and researchers together for open dialogue to streamline approval processes without sacrificing safety.
- Transparent Research Practices: Maintaining high standards of data integrity and public disclosure to build trust among patients and the broader community.
- Ongoing Ethical Reviews: Regularly reassessing the ethical implications of new technologies to address any emerging issues head on.
By carefully steering through these requirements, the healthcare community can ensure that the remarkable potential of biofabrication and early cancer detection is realized without compromising ethical or regulatory standards.
Practical Implications: How New Technologies are Shaping Patient Outcomes
As revolutionary as these technologies are, the true measure of success lies in their practical application in the clinical setting. Early detection translates to earlier, less invasive treatments that can significantly improve a patient’s quality of life. When cancer is caught in its infancy, treatment options are often more effective and less taxing on the patient’s overall health. This practical aspect of early detection via advanced modeling techniques cannot be overstated.
The following points illustrate the real-world benefits patients might one day experience as these technologies become mainstream:
- Reduced Treatment Burden: Early interventions can potentially limit the need for aggressive therapies later in the disease process.
- Improved Survival Rates: Catching cancer early increases the odds for successful treatment and long-term survival.
- Personalized Therapeutic Strategies: By tailoring treatment plans based on a patient’s unique tumor characteristics, side effects can be minimized and outcomes improved.
- Enhanced Monitoring and Follow-Up: With early detection techniques in place, clinicians can closely monitor subtle changes in patient health, leading to rapid adjustments in treatment if necessary.
These benefits represent a paradigm shift in cancer care—one where early detection and intervention are not just goals but achievable realities. As these advanced technologies are refined and gradually integrated into the standard of care, the outlook for patients facing a cancer diagnosis will increasingly be one of hope and proactive management rather than reactive intervention after the disease has fully taken hold.
Looking Back and Moving Forward: Lessons Learned from Recent Advances
Reflecting on the progress made over the past decade, it is clear that the journey toward early cancer detection has been filled with both exciting breakthroughs and nerve-racking challenges. Early experiments in 3D bioprinting paved the way for more sophisticated models, and now we stand at the tip of a new era in cancer research. Innovations stemming from institutions like OHSU have taught us that while the road is filled with tangled issues and daunting regulatory requirements, the benefits of early diagnosis and personalized treatment are too significant to ignore.
Key lessons from these advances include:
- Interdisciplinary Collaboration is Key: No single specialty can address the full range of challenges posed by early cancer detection.
- Patient-Centered Innovation: Every technological breakthrough must eventually translate to improved patient outcomes.
- Flexibility and Adaptability: As new information emerges, research methods and regulatory frameworks must evolve accordingly.
- Commitment to Ethical Standards: As we push the frontiers of biomedical research, maintaining ethical integrity must remain at the forefront of every initiative.
These lessons do more than highlight how far we have come—they also provide a roadmap for tackling the tricky parts that still lie ahead. They remind us that while the path may be loaded with problems, every step forward carries the promise of better, more targeted cancer care.
Conclusion: Embracing the Promise of Tomorrow’s Cancer Care
In conclusion, the advent of advanced biofabrication techniques, including 3D bioprinting, organoids, and organs-on-a-chip, is setting the stage for a revolution in early cancer detection. By rethinking the way we model cancer development, researchers are gaining an unprecedented look into the subtle details that govern tumor initiation. With each breakthrough, the possibility of intercepting cancer before it manifests fully becomes more tangible.
While challenges remain—in the form of regulatory hurdles, ethical concerns, and the sheer complexity of replicating human biology in the lab—the collaborative efforts of engineers, biologists, and clinicians provide a strong foundation upon which to build the future of personalized cancer care. The integration of advanced computational modeling and laboratory experiments promises to uncover the intricate sequences of events that lead from healthy cells to malignant tumors, offering a beacon of hope for patients worldwide.
Indeed, the progress so far is not just a triumph of science; it is also a testament to human ingenuity and the relentless drive to improve patient outcomes. As we continue to take a closer look at the early stages of cancer through increasingly realistic in vitro models, we are reminded that every challenge—no matter how intimidating or overwhelming—carries with it the potential for groundbreaking solutions.
The journey ahead may be filled with tricky parts and tangled issues, but one thing is certain: the future of cancer care is bright. By embracing these modern innovations and ensuring they are integrated thoughtfully and ethically into clinical practice, we edge ever closer to a reality where cancer can be intercepted at its earliest, most treatable moments—a reality where every patient has the chance to receive personalized treatment long before the disease takes hold.
As we celebrate the strides made in this field, let us also remain grounded in the reality that each innovation brings us one step closer to not only understanding but effectively combating cancer. The seamless merging of engineering, biology, and clinical care is not just revolutionizing how we see cancer—it is transforming how we treat it. With continued research, collaboration, and careful regulation, early cancer detection technologies will ultimately lead to more effective and compassionate care for patients around the globe.
In this rapidly evolving field, it is essential to keep our minds open to new ideas and our hearts committed to the welfare of every individual touched by cancer. Whether it is through 3D bioprinting, organoids, or advanced computational models, the tools of today are paving the way for the life-saving treatments of tomorrow. As we move forward, embracing both the promising advances and the challenges ahead, one thing remains clear: the future of cancer diagnosis and treatment is not only innovative—it is within our grasp.
Originally Post From https://news.ohsu.edu/2025/11/03/ohsu-researchers-identify-new-tools-for-early-cancer-detection-treatment
Read more about this topic at
OHSU researchers identify new tools for early cancer …
New Biosensors Could Revolutionize Cancer Detection