Exploring the Role of p53 Mutations in Cancer Treatment
The fascinating world of molecular oncology reminds us time and again that the tiny twists and turns in our DNA can lead to far-reaching effects. A classic example of this is the p53 gene, often lauded as the “guardian of the genome.” This gene plays an essential role in keeping our cells in check and ensuring that they remain free from dangerous mutations. Yet, when p53 mutates, it can flip from a protector into a promoter of cancer. In this opinion editorial, we take a closer look at recent research that highlights how specific p53 mutations might not only be responsible for aggressive cell proliferation but could also provide a pathway to future personalized cancer treatments.
The Guardian Turned Foe: p53’s Double-Edged Sword
It is both fascinating and a bit nerve-racking how a single gene can serve as a key part of our cellular defense system and then, under certain conditions, contribute to disease progression. The gene p53 is mutated in nearly 50% of human cancers. As a tumor suppressor, p53 ensures that cells with damaged DNA are either mended or eliminated. Yet, when p53 undergoes mutations, its ability to safeguard genomic stability is compromised. This shift is not merely a failure of protection – it can actively contribute to the dangerous, aggressive nature of cancer cells.
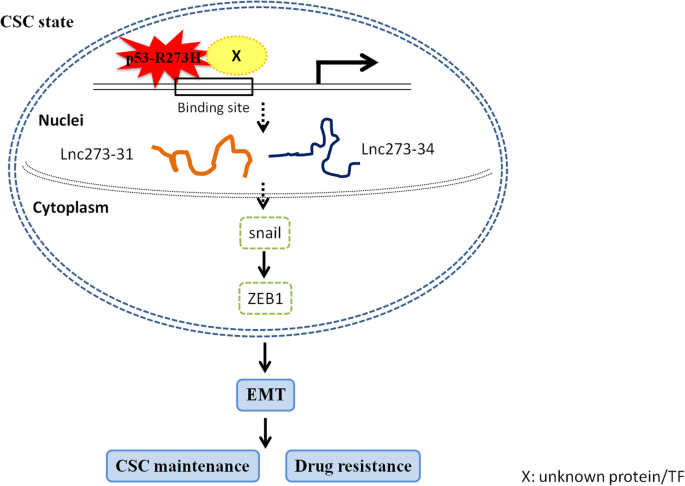
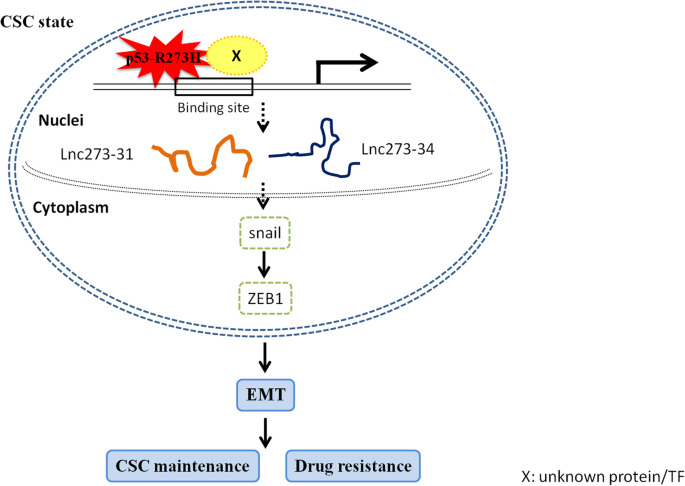
Scientists have long been intrigued by p53 mutations because they are loaded with issues that complicate both our understanding of cancer biology and the treatment approaches we might employ. Research has revealed that not all p53 mutations are equal. Some mutants, like the p53 R273H mutation, have unique behaviors in how they interact with DNA replication and immune responses, potentially opening up new treatment avenues.
Dissecting the DNA Replication Process: The Tricky Parts of p53 Mutation
One of the trickiest parts of understanding cancer biology is figuring out how molecular-level missteps contribute to the growth of tumors. Recent findings from Baylor College of Medicine have shone a light on the fact that certain p53 mutants can hijack the DNA replication machinery. Specifically, the R273H mutation causes DNA replication to fire excessively. This runaway process leads to uncontrolled cell division—a classic hallmark of cancer.
To put it simply, in a normal cell, DNA replication is a carefully regulated process. It involves multiple checkpoints and fine points that ensure each cell division happens correctly. However, when a p53 mutation like R273H is in play, this regulation is thrown into disarray. The result is a dramatic escalation in cell proliferation, which can set the stage for aggressive tumor development. The fact that this happens while simultaneously triggering other responses in the body gives rise to an intriguing possibility for therapy.
How Excessive DNA Replication Sparks Immune Responses
One might assume that unchecked cell proliferation would solely spell trouble; however, researchers discovered that the excessive DNA replication induced by the p53 R273H mutation also stokes a strong immune response. This is achieved through the activation of the cGAS-STING pathway—a core component of the body’s innate immune defenses.
The cGAS-STING pathway plays a fundamental role in detecting abnormalities within cells. When it senses the excessive DNA replication provoked by the mutant p53, it ramps up the body’s natural immune surveillance, rallying a defensive response against the burgeoning cancer cells. This phenomenon is particularly noteworthy because it suggests that p53-mutated cancers might have an unexpected vulnerability: their own rapid proliferation could be tipping off the immune system to mount an attack.
- Excessive DNA replication leads to abnormal cellular conditions.
- Activation of the cGAS-STING pathway results in a strong immune response.
- This immune activation can lead to more cancer-fighting CD8+ T cells being recruited to the tumor site.
Comparing the p53 Mutants: R273H Versus R175H
In their careful studies, the researchers at Baylor College of Medicine compared two common p53 mutations: R273H and R175H. While both mutations are known to contribute to cancer growth, they do so in markedly different ways. Such comparisons provide a nuanced look into how specific changes in the genetic code lead to very different outcomes—and why it is critical to understand these distinctive behaviors when devising treatment strategies.
The R273H mutation is unique in that it not only encourages rampant cancer cell proliferation by causing excessive DNA replication, but it also unexpectedly invokes a robust immune response. This dual effect creates a scenario where the cancer, while more aggressive in growth, also becomes more visible to the immune system. Researchers observed that in models of breast cancer, the tumors harboring the R273H mutation attracted significantly more CD8+ T cells, which are the key agents in fighting cancer cells.
Conversely, the R175H mutation, although promoting cancer progression, does not trigger this same immune activation. The absence of significant immune engagement could mean that tumors with the R175H mutation might be less responsive to certain immunotherapies. This contrast underlines the importance of appreciating the hidden complexities of each p53 mutation. By identifying which p53 variant a tumor expresses, oncologists can potentially tailor therapies to exploit the unique behavior of that mutation.
| Mutation Type | Effect on DNA Replication | Immune Response Activation | Implications for Immunotherapy |
|---|---|---|---|
| p53 R273H | Causes excessive replication | Activates cGAS-STING pathway, strong CD8+ T cell response | Potentially higher responsiveness to immune checkpoint inhibitors |
| p53 R175H | Promotes aggressive growth without replication overload | No significant immune activation | May require alternative therapeutic strategies |
Personalizing Cancer Treatment: The Future of Immunotherapy
What stands out from these findings is the exciting possibility of personalizing cancer treatment based on the specifics of the p53 mutation present. While immune checkpoint inhibitors—a class of drugs that help the immune system recognize and attack cancer cells—have already revolutionized cancer care, they do not benefit all patients equally. The research on p53 mutations offers a potential route to identify which patients might respond favorably to these immunotherapies.
In cases where the p53 R273H mutation is found, the resulting immune response could make immune checkpoint inhibitors even more effective. The body is already ramping up its defense by recruiting essential CD8+ T cells. With the help of immunotherapy, these already activated defenses could be further enhanced to better target and eliminate cancer cells. It’s a bit like finding the right key to unlock a door that leads to the body’s inherent cancer-fighting potential.
This kind of tailored approach is particularly promising because it speaks to a broader goal in modern medicine: the move toward treatments that are as unique as the patients who need them. Instead of a one-size-fits-all paradigm, the idea is to figure a path that leverages the individual genetic landscape of each tumor. The nuanced differences between p53 mutations, as highlighted in this research, present a clear opportunity to refine and improve therapeutic strategies.
Immune Checkpoint Inhibitors: A Closer Look
Immune checkpoint inhibitors work by essentially taking the brakes off the immune system. Under normal conditions, these brakes prevent an overzealous immune response that could damage healthy tissue. In cancer treatment, however, they prevent the immune system from recognizing tumor cells as threats. By disabling these brakes, checkpoint inhibitors allow the immune system to see and attack cancers more effectively.
In the context of p53 mutations, particularly R273H, the elevated immune response may enhance the efficacy of such inhibitors. When used in combination with other therapies that target the cell’s replication machinery, these drugs could potentially shift the treatment curve—turning a seemingly overwhelming tumor into a more manageable condition.
Combining DNA Replication Inhibitors with Immunotherapy
The idea of pairing immunotherapy with drugs that target DNA replication brings into focus another promising avenue in personalized medicine. The theory is straightforward: if the p53 R273H mutation triggers excessive DNA replication, then using drugs to inhibit this process could not only slow down tumor growth but also work synergistically with the immune system’s enhanced activity.
For example, in a scenario where cancer cells are replicating uncontrollably and simultaneously broadcasting distress signals via the cGAS-STING pathway, a dual treatment approach could be the answer. By simultaneously addressing the runaway cell division and bolstering the immune attack through checkpoint inhibitors, clinicians might be better positioned to tackle aggressive cancers. This strategy requires a deep understanding of each patient’s tumor biology, including the type of p53 mutation involved, the level of DNA replication activity, and the intensity of the immune response.
Such a combination therapy would be a game-changer for several reasons:
- It addresses the root cause of rapid cell growth in cancers with specific p53 mutations.
- It capitalizes on the body’s natural immune response, potentially making immunotherapy more effective.
- It allows for a more individual-centric approach to treatment, ensuring that patients receive therapies tailored to their unique disease profile.
- It could reduce treatment resistance, a problem that plagues many conventional cancer therapies.
Clinical trials in this space would involve exploring the optimal dosing regimens for both the DNA replication inhibitors and the immunotherapies, with the hope that the combination will not only arrest tumor growth but also reduce the likelihood of relapse. While the pathway to such treatments is still loaded with problems and challenges, the preliminary data turns a hopeful, promising page in the story of personalized cancer therapies.
Understanding the Immune System’s Role in Personalized Cancer Therapy
The interplay between cancer cell behavior and the immune response is a core area of study in contemporary cancer research. The immune system is adept at identifying aberrant cells, yet cancer cells have evolved strategies to hide from or even suppress immune detection. In the case of p53 mutations, however, particularly with R273H, the immune system seems to flip a switch. The overzealous cell replication indirectly leads to the activation of the cGAS-STING pathway—a critical support system for immune defense mechanisms.
This discovery invites us to re-evaluate how we think about the immune system in relation to cancer. Instead of merely trying to boost a weak immune response, we might need to recalibrate our approach to harness the existing, active immune signals elicited by specific genetic mutations. This strategy relies on understanding the hidden complexities of tumor biology—a realm where the fine points of DNA replication and immune activation interplay in unexpected ways.
It is a compelling prospect: the idea that a mutation, typically associated with poor outcomes, could be turned into an advantage by exposing the tumor to the body’s natural defenses. This strategy is not without its challenges. Deep research and meticulously planned clinical trials are required to secure these findings and fine-tune the balance between suppressing tumor growth and avoiding adverse immune reactions.
Key Considerations for Future Research
Here are several key aspects that researchers and clinicians must consider as they strive to translate these laboratory findings into practical cancer treatments:
- Patient Selection: A critical factor will be the identification of patients whose tumors harbor the specific p53 mutations, such as R273H. Diagnostic tests that accurately classify these mutations are essential.
- Treatment Timing: Determining when to administer immunotherapy and DNA replication inhibitors is essential. There may be an optimal window where the combination is most effective.
- Side Effects Management: Combining therapies always carries the risk of increased side effects. Careful monitoring and adjustment of treatment protocols will be required.
- Long-Term Outcomes: Beyond simply shrinking tumors, researchers will need to examine the long-term impact on patient survival and quality of life.
These considerations underscore the importance of ongoing research. As we continue to poke around the underlying mechanics of these mutations and their effects on the immune system, each discovery adds a valuable piece to the puzzle. The road ahead may be intimidating at times, but it is paved with the promise of a more personalized approach to cancer treatment.
Uncertainty and Optimism: Working Through Ongoing Challenges
While the research into p53 mutations brings hope, it also presents a tapestry woven with both promise and uncertainty. Cancer, at its core, is a disease marked by many tangled issues. The behavior of cancer cells, their ability to adapt, and their interaction with the immune system are all loaded with complications that researchers are still striving to fully understand.
One of the more confusing bits in current cancer research is the variable response to treatments such as immune checkpoint inhibitors. Not all tumors behave the same way, and even among those with the same p53 mutation, subtle differences in cell behavior and replication dynamics can lead to different clinical outcomes. This variability necessitates an approach where treatment is continually adapted based on real-time patient response. It is akin to finding your way through a maze where the walls keep shifting—a nerve-racking experience for both patients and clinicians alike.
Even amid these challenges, there is optimism in the research community. The possibility of using the very mutations that once spelled doom for patients as targets for cutting-edge therapies exemplifies the innovative spirit of modern medicine. By taking a closer look at each detailed mechanism—from aberrant DNA replication to immune system activation—scientists are better equipped to design strategies that will ultimately improve outcomes.
Balancing Risks and Rewards in Experimental Treatments
Developing new treatments is never a straightforward path. It involves weighing the potential benefits against the possible risks and side effects. In this case, the combination of immunotherapy with drugs targeting DNA replication carries its own set of challenges:
- There is the possibility of an overactive immune response, which in some cases could lead to autoimmune reactions.
- Suppressing DNA replication too much might adversely impact healthy cells, particularly those that rapidly divide, such as cells in the digestive tract or bone marrow.
- Optimizing the dosage of both drugs to achieve the best synergy without tipping the scales too far in one direction requires extensive clinical testing.
Finding this balance is critical. The ultimate goal is to craft a treatment that not only halts the progression of the cancer but does so without placing an undue burden on the patient’s overall health. Researchers are already developing new compounds and treatment protocols that might one day offer a tailored solution for those carrying the p53 R273H mutation, among other genetic markers.
Impact on Future Cancer Treatment Paradigms
The implications of this research reach far beyond the laboratory. As the field moves toward personalized medicine, the ability to identify and exploit the unique characteristics of each tumor becomes a must-have tool for oncologists. The emerging evidence that different p53 mutations can lead to varied immune responses and treatment outcomes is a clear signal that the future of cancer care will be more individualized.
Future therapies might involve a comprehensive diagnostic workup that not only sequences the tumor’s genome but also assesses the level of immune activation and DNA replication activity. Imagine a scenario where, upon diagnosis, a patient receives a customized treatment plan tailored to the precise genetic alterations within their tumor. Such an approach would certainly represent a watershed moment in our collective fight against cancer.
This shift toward individualized therapy is already underway in many aspects of medicine. With advancements in genomic testing, personalized cancer vaccines, and targeted therapies, we are starting to see the pieces of a much larger puzzle come together. Each new discovery in this realm not only expands our scientific horizons but also offers tangible hope to patients and families impacted by this complex disease.
Standardizing Diagnostic Protocols for p53 Mutations
In order to make personalized treatment the mainstream approach, a few practical steps will need to be taken within the clinical setting:
- Enhanced Genetic Screening: Hospitals and cancer centers will need to adopt comprehensive genomic screening protocols. This ensures that mutations such as p53 R273H and R175H are identified early and accurately.
- Training Clinicians: Medical professionals must be well-versed in interpreting genetic data and understanding its implications on treatment decisions. Continued education and multidisciplinary collaboration are key.
- Integrated Care Teams: Specialists in oncology, immunology, and genomics will need to work together to craft individualized treatment plans that address both the cancer’s aggressive growth and its immune environment.
- Patient Engagement: It is essential to educate patients about the role of genetic mutations in their disease so that they can take an active role in treatment decisions.
Addressing these aspects early on can enhance the overall effectiveness of personalized medicine approaches, ensuring that every patient gets the best possible care tailored to their unique biologic profile.
Looking Ahead: Research, Trials, and Patient Impact
The translation of laboratory research into clinical practice is always a journey filled with both breakthroughs and setbacks. The studies illuminating the behavior of p53 mutations provide an optimistic glimpse into what might be possible in the future. However, significant research and clinical trials lie ahead before these insights can be seamlessly integrated into standard care protocols.
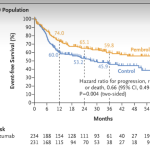
There is every reason to be cautiously optimistic. Ongoing clinical trials are already testing the efficacy of combining multiple treatment modalities, and early results are promising. More importantly, these studies underscore the fact that a one-size-fits-all strategy is no longer acceptable in the realm of oncology. By understanding the specific little twists in each cancer’s genetic makeup, the medical community is poised to deliver more targeted and effective treatments.
Patients stand to benefit the most from these innovations. Imagine receiving a treatment plan that takes into account not just the location and size of your tumor, but the specific genetic quirks that define its behavior. Such precision not only enhances treatment efficacy but also minimizes the risk of side effects, leading to better overall quality of life.
Opportunities for Multi-Disciplinary Collaboration
The future of cancer treatment relies heavily on interdisciplinary collaboration. The integration of basic science, clinical research, and advanced genomic diagnostics will be the backbone of any successful personalized therapy strategy. Key opportunities include:
- Collaboration between molecular biologists and immunologists to further understand how p53-related immune responses can be harnessed.
- Partnerships between pharmaceutical companies and academic centers to develop new drugs that precisely target the overactive DNA replication seen in certain p53 mutations.
- Improving data sharing between institutions globally, ensuring that findings are quickly translated into clinical protocols.
- Enhanced patient registries to track treatment responses over the long haul, thereby guiding future research and therapy adjustments.
These steps are essential for building a more resilient, adaptive, and effective cancer care ecosystem. By bridging the gap between the lab bench and the patient bedside, we can better manage the tangled issues of cancer—turning potential setbacks into novel treatment opportunities.
Final Thoughts: Balancing Innovation with Patient Care
The exploration of p53 mutations and their impact on cancer growth represents a pivotal moment in oncology. While the path forward is full of challenges and occasional setbacks, the insights gained from research on mutations like R273H and R175H offer a more detailed look into the fine points of cancer biology. It is an evolving field where new ideas are constantly emerging, and every discovery brings us a step closer to truly personalized cancer care.
In the grand tapestry of cancer research, each thread—from understanding the nitty-gritty of DNA replication to harnessing the body’s immune response—plays a critical role in the overall picture. The possibility that a mutation once considered purely detrimental might actually guide us in confronting cancer more effectively is both inspiring and transformative.
As we steer through these exciting developments, a few key principles should guide our journey:
- Remain open to new ideas while carefully considering risks and rewards.
- Invest in robust, standardized diagnostic procedures that can accurately identify specific genetic mutations.
- Foster interdisciplinary collaboration that bridges research with clinical application.
- Prioritize patient engagement and education, ensuring that individuals understand the factors influencing their treatment options.
The research on p53 mutations is a vivid reminder that modern medicine is constantly evolving. As we figure a path through the challenging maze of cancer biology, every breakthrough—no matter how small—brings us closer to a future where personalized, precision-based treatments become the norm rather than the exception.
For patients and clinicians alike, these developments represent more than just the promise of new drugs or therapies. They offer hope that, someday, cancer treatment will be as unique as the person facing it—a tailored journey that considers every subtle detail of an individual’s genetic makeup and immune profile.
Embracing the Future with Both Caution and Hope
When considering the future of cancer treatment based on p53 mutation research, it is important to balance innovation with grounded, patient-centered care. The science is paving the way for individualized intervention strategies that harness both immunotherapy and drugs targeting DNA replication. However, as with all cutting-edge science, it is crucial to be mindful of the unexpected twists and turns that can emerge along the way.
Clinical trials and further research are necessary to determine the safest and most effective methodologies for applying these insights in everyday practice. While initial findings are indeed promising, translating this knowledge into widely available treatment protocols requires collaborative effort and time. The delicate balance of speeding innovation while ensuring patient safety is a continual challenge—one that the medical community is committed to tackling head-on.
Conclusion: A New Era in Personalized Oncology
The journey from understanding the p53 gene’s role in maintaining genomic stability to manipulating its mutated forms for therapeutic gain exemplifies modern medicine’s dynamic nature. Research into the contrasting effects of p53 mutations such as R273H and R175H not only deepens our scientific understanding but also opens the door to innovative, personalized approaches to cancer treatment.
By taking a closer look at the tricky parts of DNA replication and the hidden signals that mobilize the immune system, scientists are beginning to chart a course through the maze of cancer biology. This course, though loaded with challenges, offers a promising pathway to treatments that are as unique as the tumors they target.
In our ongoing struggle against cancer, embracing the promise of personalized medicine means understanding each subtle difference, each small twist in the genetic code. It is about combining the power of immunotherapy with targeted therapies tailored to an individual’s genetic profile. As we move forward, doctors and researchers must continue to collaborate, innovate, and remain committed to patient-centered care.
Indeed, the future of oncology may well rest on our ability to translate these detailed laboratory insights into practical, effective therapies that provide hope to countless patients. With each step taken—from improved diagnostic protocols to the development of combination therapies—we move closer to a day when the diagnosis of cancer is met not with despair, but with a tailored plan of action that optimizes every available tool.
Ultimately, these advances remind us that even in the face of overwhelming challenges, science continues to unravel the tangled issues of disease, guiding us toward a future where personalized, effective, and compassionate cancer care is a reality for all.
Originally Post From https://blogs.bcm.edu/2025/11/11/from-the-labs-p53-mutant-r273h-opens-new-avenues-for-future-personalized-cancer-treatment/
Read more about this topic at
Mutant p53 exploits enhancers to elevate immunosuppressive …
Mutant p53 exploits enhancers to elevate …