Early Warning Signs: Understanding Paraneoplastic Neurologic Syndromes in Testicular Germ Cell Tumors
The recent study highlighting that patients with testicular germ cell tumors (TGCTs) often show signs of paraneoplastic neurologic syndromes (PNSs) before their cancer diagnosis presents a thought-provoking look into the tangled issues connecting neurology and oncology. As an editor at an online healthcare journal with expertise in modern and alternative medicine, nutrition, disease conditions, and fitness, I find the results both intriguing and challenging. The study invites us to take a closer look at the early symptoms that could signal an underlying malignancy—and to consider how early detection might reduce long-term neurological disabilities and enhance overall cancer treatment outcomes.
In an era when healthcare is increasingly focused on early diagnosis and personalized treatment, the findings from this retrospective cohort study serve as a super important reminder that some of the most subtle signs can be the key that unlocks earlier intervention. As we explore the topic here, I will dig into the research findings and consider the implications for both patients and practitioners, while also discussing the tricky parts of interpreting these signals in everyday clinical practice.
Early Detection and the Role of Neural Autoantibodies
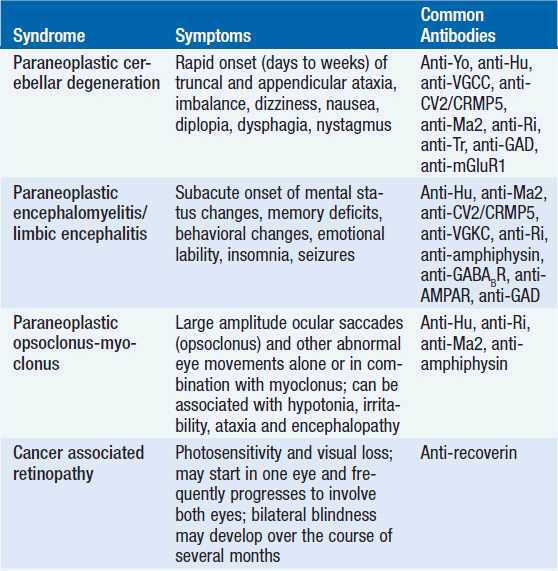
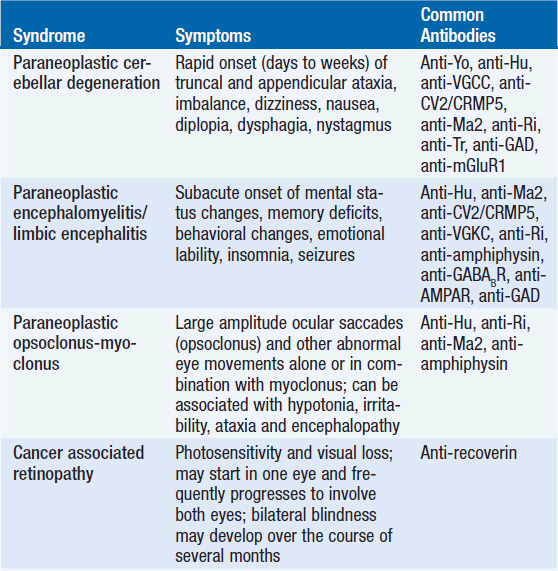
The study showed that nearly 80% of patients with TGCT experienced neurological symptoms such as ataxia, diplopia, sensorineural hearing loss, and vertigo before their cancer diagnoses. These signs are not merely coincidental; they are often linked to neural autoantibodies including Kelch-like protein 11 (KLHL11-IgG), leucine zipper 4 (LUZP4-IgG), and Ma2-IgG.
These neural autoantibodies can serve as essential biomarkers, alerting clinicians to the possibility of an underlying, yet undiagnosed, TGCT. For example, KLHL11-IgG was the most frequently detected antibody among the studied cases. In many instances, its presence was associated with specific neurological issues such as sensorineural hearing loss and tinnitus. Meanwhile, Ma2-IgG showed strong links to seizures and sleep disturbances. Thus, when neurologists or oncologists come across these clues, they can figure a path toward further investigations into both the neurologic and oncologic aspects of the patient’s condition.
Identifying the Subtle Details in Antibody Profiles
Breaking down the antibody profiles reveals several small distinctions that are crucial for timely diagnosis. The research underscores the following points:
- KLHL11-IgG: Often found in patients with seminomas, this antibody was linked to symptoms such as sensorineural hearing loss and tinnitus. Its association with such sensory deficits points to the importance of paying attention to even minor changes in hearing and balance.
- LUZP4-IgG: Frequently co-detected with KLHL11-IgG, LUZP4-IgG adds another piece to the complex puzzle of PNS presentations in TGCT. The combined presence of these antibodies could help refine the diagnostic process.
- Ma2-IgG: Mostly associated with nonseminomatous germ cell tumors (NSGCTs), Ma2-IgG was linked to seizures and sleep disturbances. Recognizing these associations is key to addressing the nerve-racking symptoms early on.
Each of these biomarkers represents a fine point in the broader context of cancer-associated neurologic disorders. Their detection is not just a laboratory curiosity—it is a call for action for clinicians to consider broader oncological assessments when puzzling over otherwise unexplained neurologic symptoms.
Challenges in Diagnosing Overlapping Conditions
One of the main challenges in clinical practice is dealing with the confusing bits where neurologic symptoms overlap with cancer indicators. The study points out that while many patients eventually had favorable oncological outcomes, a significant number still dealt with persistent neurological issues. This disconnect between cancer prognosis and long-term neurologic function illustrates the tricky parts of managing these dual diagnoses.
In many cases, the neurological symptoms developed well before the cancer was identified, creating an intimidating scenario for both patients and doctors alike. The symptoms of ataxia and diplopia, for example, might be misattributed to other more common neurological disorders if the possibility of an underlying TGCT is not considered. This overlap can lead to delays in both cancer screening and the initiation of appropriate neurologic treatment.
Understanding the Twists and Turns: A Closer Look at Patient Data
Table 1 below summarizes some key findings from the patient data discussed in the study:
| Neurological Symptom | Frequency among Patients | Associated Autoantibodies | Tumor Type Connection |
|---|---|---|---|
| Ataxia | 31 patients | KLHL11-IgG primarily | Seminomas |
| Diplopia | 29 patients | KLHL11-IgG and others | Seminomas |
| Sensorineural Hearing Loss | 22 patients | KLHL11-IgG | Seminomas |
| Vertigo | 20 patients | Mixed antibody presence | Seminomas |
This table not only underscores the frequency of neurologic symptoms but also highlights the tangled issues in correlating these symptoms with specific tumor types and antibody profiles. The small distinctions observed in the autoantibody associations provide critical clues that could lead to earlier cancer detection and tailored treatment strategies.
Interdisciplinary Coordination: A Must-Have Approach
The research makes it clear that managing cases that involve both TGCT and PNS is not a task for any single specialist. Instead, it calls for a collaborative approach, involving neurologists, oncologists, immunologists, and even primary care physicians. Such a multifaceted team can better work through the tricky parts of diagnosing and treating these overlapping conditions, ensuring that both neurologic and oncologic aspects receive the attention they require.
Working through these dual diagnoses requires more than just recognizing the initial red flags; it demands a systematic screening protocol. In community-based settings, where resources might be limited compared to large academic institutions, practical screening algorithms for detecting neural autoantibodies are critical. These protocols can help physicians get around the complicated pieces of diagnosis by showing them when to suspect TGCT in the presence of otherwise inexplicable neurologic symptoms.
Creating a Screening Algorithm: Key Components
When considering the implementation of a screening algorithm for PNS among TGCT patients, several key steps should be taken:
- Initial Symptom Identification: Physicians must be aware that symptoms like ataxia, diplopia, sensorineural hearing loss, and vertigo could be the first hints of a deeper problem.
- Autoantibody Testing: Testing for KLHL11-IgG, LUZP4-IgG, and Ma2-IgG should be considered in patients who present with unexplained neurologic symptoms.
- Comprehensive Imaging Studies: Once neural autoantibodies are detected, imaging and additional diagnostic tests must be rapidly deployed to rule out or confirm the presence of TGCT.
- Interdisciplinary Consultation: A team-based approach should be activated early in the diagnostic process to collaborate on the best path forward.
By following the above steps, clinicians can more effectively dig into the early signs of TGCTs, sidestepping some of the nerve-racking delays that can lead to persistent neurological issues even after cancer remission.
Patient Outcomes and Long-Term Considerations
While the study revealed that 80% of patients achieved either cure or remission from their TGCTs, it is concerning that many continued to suffer from long-term neurological disabilities. Approximately 16% of patients showed improvement in their PNSs, while 43% saw stabilization and 41% experienced progression despite immunomodulatory treatment. These numbers highlight that even when the cancer itself is managed successfully, the neurological impact can continue to burden patients.
The persistence of neurologic symptoms poses tricky parts and subtle details for post-treatment care and rehabilitation. For patients, the journey does not end with remission; rather, it transitions into managing persistent issues that can affect quality of life, cognitive function, and even emotional well-being. The overlapping effects of a resolved cancer diagnosis and ongoing neurological disability require patient care strategies that extend well beyond the initial treatment phase.
Developing Long-Term Management Plans
Long-term management of patients with TGCT-associated PNS should include a multifaceted care plan:
- Neurologic Rehabilitation: Tailored therapies to improve motor skills, balance, hearing, and vision can offer significant benefits.
- Chronic Pain Management: For those suffering from nerve-related pain, dedicated pain management strategies—including both medical and alternative approaches—are essential.
- Psychosocial Support: Persistent neurologic symptoms can be overwhelming, leading to anxiety and depression. Access to mental health support is a key component of overall care.
- Regular Follow-Up: Continuous monitoring is necessary to catch any changes in neurologic function, ensuring timely adjustments to the treatment plan.
With these measures in place, patients can better manage the long-term consequences of their condition, even when the initial oncological outcomes are favorable. This process is as much about maintaining quality of life as it is about addressing medical symptoms.
The Impact of Interdisciplinary Research on Clinical Practice
The study at hand is a clear testament to the benefits of interdisciplinary research. By involving experts from neurology, oncology, and immunology, researchers were able to uncover significant associations between neural autoantibodies and specific tumor types. This approach ensures that the subtle details—such as the exact prevalence of KLHL11-IgG in patients with seminomas or the strong correlation of Ma2-IgG with NSGCTs—are not lost in a siloed examination of illnesses.
When clinicians from different specialties work together, they can pool their expertise to better understand the complicated pieces of patient presentations. This coordination is not just beneficial—it is essential. Treating the off-putting mix of cancer-related and neurological symptoms requires all hands on deck, ensuring that no part of the patient’s condition is overlooked.
Bridging the Gaps: How Teams Can Improve Patient Care
Improved patient outcomes are often a direct result of successful teamwork in the medical field. Here are some strategies that can help bridge the gaps between specialties:
- Regular Multidisciplinary Meetings: Bringing together neurologists, oncologists, immunologists, and radiologists on a regular basis fosters better communication and collaborative decision-making.
- Shared Electronic Health Records: When all team members have access to a patient’s complete medical history and test results, it becomes easier to pick up on the little details that might otherwise be missed.
- Cross-Training Opportunities: Encouraging doctors to attend conferences and seminars outside their primary specialty can ‘open up’ their perspective and help them better understand the full spectrum of issues presented by conditions like TGCT-associated PNS.
- Patient-Centered Care Models: Ensuring that care plans are designed around the patient’s overall well-being, rather than a single aspect of their disease, helps in managing the full range of symptoms and enhancing quality of life.
When these teamwork strategies are implemented, healthcare professionals can more efficiently figure a path through the twists and turns of diagnosis and treatment. In doing so, they not only address the immediate health concerns but also lay the groundwork for improved long-term outcomes.
The Role of Immunomodulatory Treatment: Opportunities and Limitations
While the majority of patients demonstrated positive responses to cancer treatments, the story is more complex when it comes to managing PNS. Only a small percentage of patients experienced improvement in their neurological symptoms, despite receiving immunomodulatory therapy. This discrepancy raises important questions about the nature of immune system involvement in paraneoplastic syndromes.
Immunomodulatory treatments are designed to adjust or curb the immune response. In cases of PNS, these treatments aim to mitigate the immune system’s attack on neural tissues. However, the outcomes have been mixed. With nearly 41% of patients seeing progression in their neurologic symptoms, it is clear that while these therapies might be effective in some cases, there is still a lot of work to be done in understanding and managing the subtle details of immune response in this setting.
Opportunities for Advancing Immunotherapy
Advancements in immunotherapy hold promise for offering more targeted and effective treatments in the future. Some areas ripe for further exploration include:
- Personalized Medicine: By tailoring immunomodulatory treatments to each patient’s specific antibody profile, clinicians may be able to improve outcomes.
- Biomarker Research: Continued research into the roles of KLHL11-IgG, LUZP4-IgG, and Ma2-IgG might offer insights into why some patients respond better than others.
- Combination Therapies: Using immunotherapy in conjunction with other treatment modalities, such as targeted chemotherapy or radiation, could provide a more comprehensive approach to managing both cancer and neurologic symptoms.
- Longitudinal Studies: Further research over extended periods can help clarify how neurological symptoms evolve over time and which interventions are most effective during different stages.
These research opportunities are super important. They represent pathways to not only improve survival rates but also to enhance quality of life by reducing long-term neurological disabilities. As the medical community continues to dig into the challenges of treating PNS in the context of TGCT, patient care standards are likely to evolve significantly in the coming years.
Community and Nonacademic Settings: Practical Implications
One notable takeaway from the study is the emphasis on the need for practical screening algorithms in community settings. In many nonacademic hospitals and clinics, physicians do not have immediate access to the full range of advanced testing used in major research centers. This reality necessitates the development of streamlined, easy-to-use protocols that can detect warning signs of PNS even with limited resources.
For community practitioners, the following practical steps can be implemented:
- Heightened Awareness: Educate local healthcare providers about the early signs of PNS and its association with TGCT. This includes recognizing symptoms such as ataxia, diplopia, sensorineural hearing loss, and vertigo.
- Basic Autoantibody Screening: Establish protocols for initial screening of neural autoantibodies, even if full-scale laboratory testing is not available in-office.
- Referral Networks: Develop a robust referral system for patients who exhibit these symptoms so that they can receive more specialized testing and treatment at larger centers.
- Patient Education: Inform patients about the potential significance of their neurological symptoms. When patients know what to watch out for, they are more likely to report changes early, allowing for earlier intervention.
Implementing these measures can help bridge the gap between cutting-edge research and day-to-day clinical practice. With a stronger grasp of the subtle signals that indicate underlying TGCT, community healthcare workers can substantially reduce the probability of overlooking early symptoms of PNS.
Exploring Alternative and Complementary Approaches
While conventional treatment strategies focus heavily on immunomodulatory therapies and surgical interventions for TGCT, there is also growing interest in alternative and complementary therapies. These approaches can offer additional support, particularly in managing the long-term neurologic disabilities that may persist after cancer remission.
Alternative therapies such as acupuncture, nutritional support, mindfulness, and physical rehabilitation can help address the secondary symptoms of PNS. For example, acupuncture may help alleviate chronic pain, while nutritional counseling can support overall neurological function. Combining these approaches with conventional medicine encourages a more holistic view of patient care.
Integrating Complementary Therapies into Conventional Treatment
There are several ways that alternative remedies can augment the standard care of TGCT and PNS patients:
- Acupuncture and Physical Therapy: These interventions can help improve balance and coordination in patients suffering from ataxia and vertigo.
- Nutritional Support: A balanced diet rich in omega-3 fatty acids, vitamins, and antioxidants can support nerve health and reduce inflammation.
- Mindfulness and Stress Reduction: Techniques such as meditation and yoga can help manage the anxiety and overwhelming stress associated with chronic neurologic issues.
- Patient Counseling: Psychological support, including group therapy or individual counseling, can be key in helping patients deal with the far-reaching impact of both cancer and its neurological sequelae.
The integration of these complementary therapies can be seen as another tool in the overall strategy for tackling the multifaceted issues associated with TGCT and PNS. Through a supportive, patient-focused approach, healthcare providers can help mitigate the nerve-racking effects of long-term neurological complications.
The Bigger Picture: Implications for Future Research and Clinical Practice
In light of the significant connections between TGCT and PNS explored in the study, several broader implications emerge for both future research and clinical practice. One key message is the necessity of comprehensive, multidisciplinary studies that shed light on the tiny details of how immune responses interact with both cancer and the nervous system.
Future research should continue to explore:
- The Role of Genetic Factors: Understanding the genetic predispositions that might influence the development of neural autoantibodies can further refine patient screening and individualized treatment strategies.
- Advanced Imaging Techniques: Innovations in imaging and diagnostic technologies will help clinicians better visualize the fine points of neural damage and better correlate these changes with antibody profiles.
- Long-Term Outcome Studies: Comprehensive follow-up studies are needed to assess how early recognition of PNS can impact long-term neurological function, quality of life, and survival rates.
- Interventional Trials: Clinical trials that test new immunomodulatory and combined treatment strategies could pave the way for more effective therapies that address both TGCT and its neurological consequences simultaneously.
These research areas are not only essential for pushing the boundaries of medical science but are also key to ensuring that improvements in treatment translate into real-world benefits for patients. They remind us that amidst the dizzying twists and turns of modern medicine, every small detail matters.
Conclusion: Bridging the Gap Between Neurology and Oncology
The relationship between paraneoplastic neurologic syndromes and testicular germ cell tumors is a vivid reminder of the hidden connections that can exist between seemingly separate medical conditions. With nearly 80% of patients displaying neurological symptoms before a TGCT diagnosis, the findings underscore the super important need for clinicians to dig into these early warning signs.
Through the enhanced detection of neural autoantibodies, improved interdisciplinary coordination, and the integration of complementary therapies, the medical community can work to figure a path toward earlier diagnosis and more effective management. Although the journey is full of daunting twists and turns, every small advance made in our understanding provides hope for reducing long-term neurological disabilities and enhancing the overall quality of life for patients.
As we look into the future, a collaborative approach remains the key to addressing these complicated pieces. Clinicians, researchers, and healthcare providers must work together to develop practical screening algorithms, tailor interventions based on nuanced antibody profiles, and ensure that both oncological and neurological issues receive the tailored care they deserve.
The study not only reveals the nerve-racking reality of persistent neurological issues even after successful cancer outcomes—but also emphasizes that early detection and appropriate intervention can make a real difference. By continuing to work through the confusing bits of this intertwined condition, we stand a better chance of turning early warnings into successful prognoses and improved patient wellbeing.
Ultimately, the message for both healthcare professionals and patients is clear: pay close attention to the subtle details. When unusual neurological symptoms arise, consider them as potential signposts—not only of a neurological disorder but perhaps of an underlying cancer. And in this shared quest for better health outcomes, every small step forward is a victory for both science and patient care.
Originally Post From https://www.neurologylive.com/view/paraneoplastic-neurologic-syndromes-present-before-testicular-germ-cell-tumor-diagnosis-study-shows
Read more about this topic at
Paraneoplastic Syndrome: Symptom, Causes and Treatment
Paraneoplastic syndromes of the nervous system