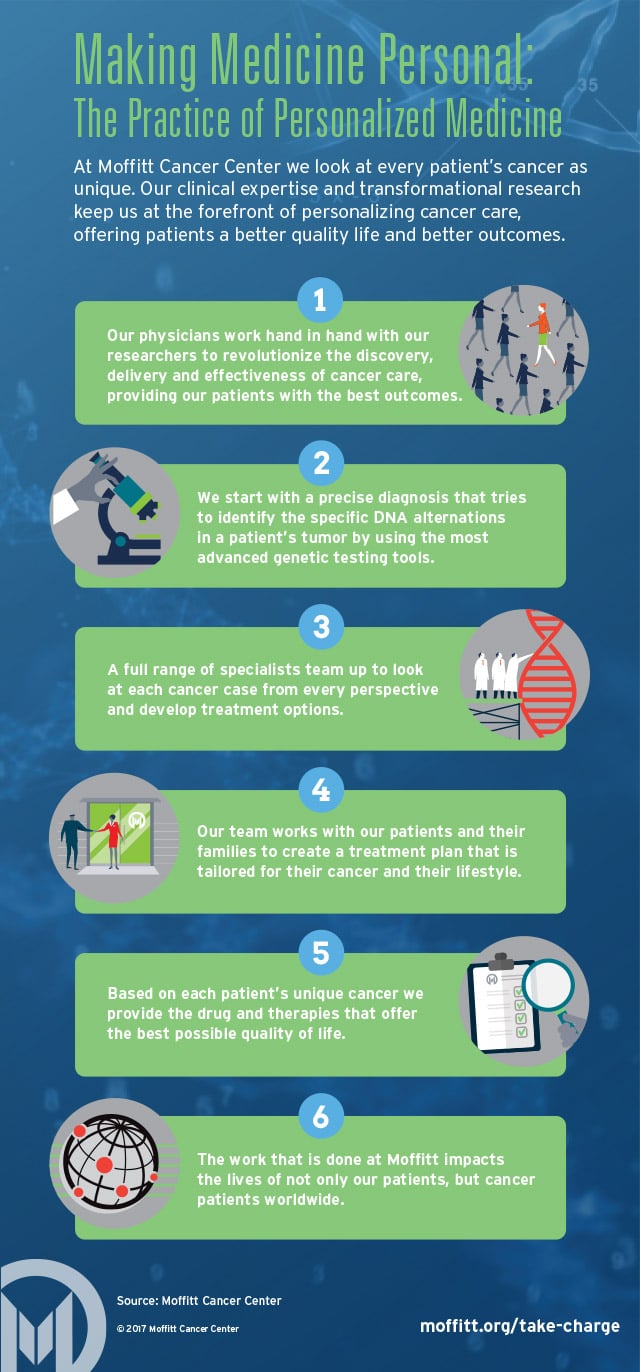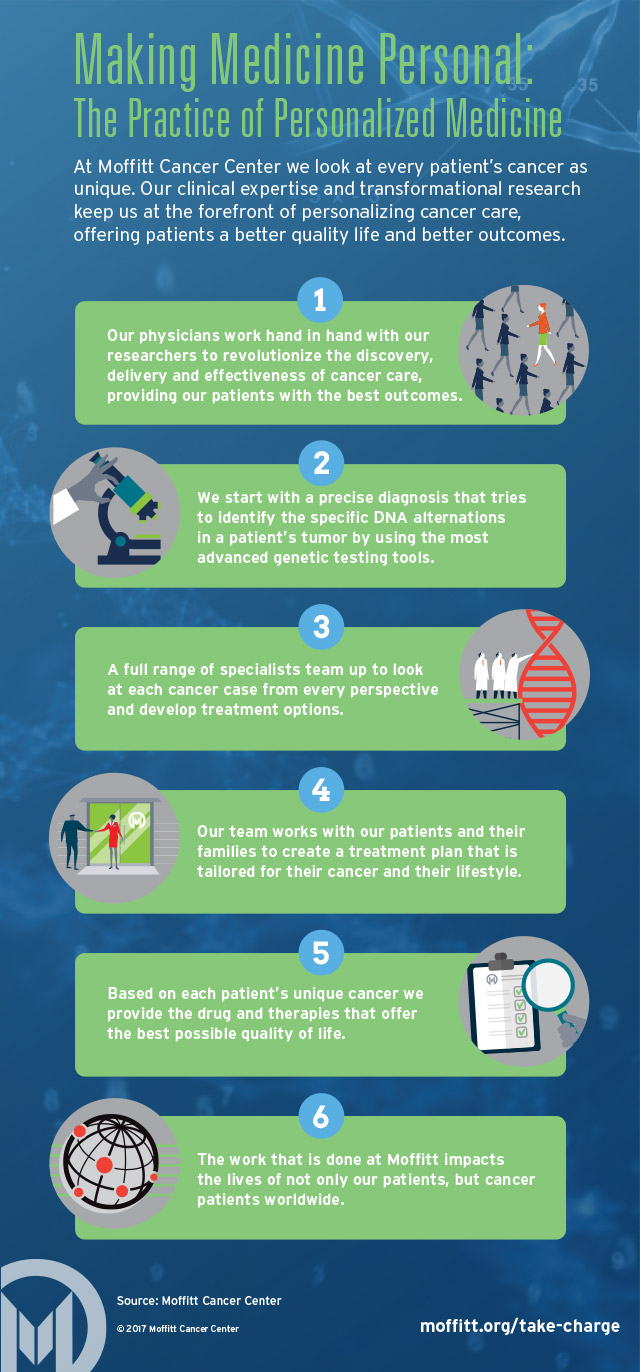
Personalizing Cancer Treatment: A New Frontier in Oncology
The landscape of cancer treatment is shifting from traditional one-size-fits-all methods toward a more personalized solution. Recent developments in computational analysis techniques have opened up a whole new chapter in the fight against cancer, one where the unique genetic profile of each tumor becomes the blueprint for effective therapy. This editorial reflects on how advanced methods such as VIPER (Virtual Inference of Protein activity Enriched by Regulon) are reshaping our approach to cancer treatment while exploring the many challenges and opportunities that come with this progressive path.
In today’s world, where the medical community is working through tangled issues in understanding tumor biology, the movement toward personalized therapy represents not only a technological leap but also a change in mindset. As oncologists and researchers jump in to embrace innovative strategies, the discussion now turns to how computational models can reliably predict drug responses and tailor treatments to the individual profiles of cancer patients.
Computational Analysis Techniques in Oncology: Paving the Way for Tailored Therapies
Traditionally, cancer treatment has been dominated by broad strategies such as chemotherapy. These methods, which rely on targeting rapidly dividing cells, do not account for the fine points and subtle details of the genetic alterations that drive tumor growth. Over time, the limitations of these approaches have become more evident—especially when considering the sophisticated biological heterogeneity among tumors, even within the same cancer type.
Advancements in computational analysis have changed the game by introducing methods that can dig into the unique aspects of each tumor. One such method, VIPER, stands out by its ability to measure the activity of oncoproteins—the proteins that tumors hijack to fuel their uncontrolled growth. In simple terms, think of oncoproteins as the stuck gas pedal in a car; even if two vehicles look the same from the outside, the issues hidden under the hood may be entirely different. VIPER essentially helps to figure a path through the tangled issues of protein activity that contribute to cancer progression.
How VIPER Works: A Closer Look at the Technology
VIPER uses gene expression data from the entire transcriptome—a complete snapshot of all messenger RNA strands—to determine the activity levels of numerous oncoproteins in a given tumor sample. The process involves analyzing the regulon of each oncoprotein, which is essentially every gene that these proteins control. By piecing together this network of interactions, VIPER provides a dynamic picture of tumor behavior that goes beyond what standard mutation analyses can show.
This method marks a departure from older techniques that only examined the genetic blueprint—the mutation status—without considering whether the actual protein ‘gas pedals’ were active. As such, VIPER reflects a critical shift: instead of merely cataloging the gene mutations, it actively predicts which mutated or unmutated proteins are playing key roles in tumor progression.
Comparing VIPER to Traditional Mutation-Based Approaches
One of the most compelling reasons for adopting VIPER is its superior predictive power. In a recent study focusing on lung cancer cell lines, VIPER was used to predict the efficacy of an EGFR inhibitor—a drug designed to target the well-known EGFR oncoprotein. Traditional mutation-based methods failed to accurately forecast which cell lines would respond to treatment because they missed the real-time activity of oncoproteins. In contrast, VIPER’s predictions were in line with the actual results observed in cell survival studies.
Below is a simplified table that highlights the distinction between the two approaches:
| Approach | Basis for Prediction | Predictive Focus | Outcome |
|---|---|---|---|
| Mutation-Based Analysis | Genetic blueprint | Presence or absence of mutations | Often overlooks active signaling pathways |
| VIPER Approach | Gene expression and regulon activity | Actual oncoprotein activity patterns | Better prediction of drug response, regardless of mutation presence |
This table illustrates how the traditional method tends to fall short when the situation is loaded with issues that the genetic blueprint alone cannot explain. VIPER, by contrast, takes a more dynamic view—focusing on the actual biological processes that drive tumor growth.
Understanding Tumor Genetics: The Case of Oncoproteins and EGFR
It is widely acknowledged that cancers are born out of genetic alterations, but not all mutations are created equal. In some cases, mutations result in the activation of proteins that play a fundamental role in the signaling pathways driving uncontrolled cell division. These proteins, known as oncoproteins, can effectively act as accelerators for tumor growth. The case of EGFR in lung cancer is one of the most studied examples in modern oncology.
EGFR (Epidermal Growth Factor Receptor) has been the target of numerous drugs designed to interfere with its signaling, but success rates have varied. Part of the issue lies in the fact that EGFR mutations alone do not paint the full picture. In many instances, cells with mutated EGFR do not respond to treatment if the overall activity of the protein is low, and conversely, cells with normal EGFR genes may respond well if the activity is high.
Using VIPER, researchers have observed that the actual activity of EGFR is a more accurate indicator of drug sensitivity than the mutation status alone. This finer distinction is essential for designing treatment regimens that are tailored to a patient’s unique tumor profile.
The Dual Role of Genetic Mutations and Protein Activity
Understanding cancer requires working through a series of layered questions: Is the mutation present causing a malfunction in protein activity? And if so, how does that malfunction translate into faster tumor growth? These questions highlight the intertwined roles of genetic mutations and the subsequent protein dynamics in contributing to cancer’s progression.
When it comes to indicating treatment strategies, knowing which mutated genes are actually active is key. Here’s a brief outline to get a grasp on the issue:
- Genetic Mutations: Provide a static picture of potential malfunction.
- Protein Activity: Offers a dynamic insight into the tumor’s current state.
- Combined Analysis: Integrating both can offer a robust indicator for drug efficacy.
This layered analysis is critical because it reveals that the presence of a mutation alone does not automatically make a tumor sensitive to a particular drug. In many ways, VIPER helps to untangle the confusing bits and narrow down the subtle parts that truly matter in making treatment decisions.
The Role of Personalized Medicine: Perspectives from VIPER Research
The drive toward personalized medicine is more than just a trend; it is a response to the overwhelming complexity of cancer biology. Modern tools like VIPER represent a step away from a uniform treatment strategy toward crafting bespoke solutions based on the unique narrative of each tumor.
Oncology has been on edge for a long time with the challenges posed by the hidden complexities of tumor behavior. Researchers are now beginning to successfully link oncoproteins to specific gene mutations and their subsequent functional roles. However, the twin challenges remain: determining which oncoproteins are activated in any given tumor and identifying which of these activations are truly pushing the tumor to grow. VIPER stands out as it attempts to provide solutions to both these highly intricate questions.
Key Insights from VIPER Research in Personalized Cancer Treatments
Several interesting points emerge from recent research using VIPER, leading to a deeper understanding of how personalized treatments can be optimized. These insights include:
- Enhanced Drug Response Prediction: By assessing oncoprotein activity, VIPER makes it possible to predict the effectiveness of a drug more accurately than relying on mutation status alone.
- Broad Application Potential: VIPER’s analytical method is applicable to any tumor for which gene expression data can be acquired, regardless of the cancer type.
- Customized Treatment Options: The technique could help clinicians identify non-mutated but active proteins that are contributing to tumor growth, thereby expanding the spectrum of potential target proteins.
- Reducing Uncertainty: For patients, a more precise prediction of drug response means less guesswork in treatment selection.
VIPER’s ability to predict tumor response by taking a closer look at the nitty-gritty of oncoprotein activity is an essential advance in the field of precision medicine. By moving beyond static mutation data and integrating the small distinctions regarding actual protein function, this approach could eventually reduce the trial-and-error method that has long been a part of cancer therapy.
Evaluating Drug Responses Using VIPER: A Case Study in Lung Cancer
A recent study on lung cancer cell lines offers compelling evidence for the advantages of using VIPER in personalized medicine. In this study, researchers focused on the EGFR oncoprotein—long known for its involvement in promoting cancer cell proliferation when activated. They assessed the response of 79 lung cancer cell lines to an EGFR inhibitor, comparing the predictions made by conventional mutation-based methods with those made by VIPER.
The experiment involved two key evaluation steps:
- Prediction Stage: VIPER analyzed the gene expression data to predict which cell lines exhibited high EGFR activity versus low activity.
- Validation Stage: The cell lines were then treated with an EGFR inhibitor, and their survival was monitored to see if the predictions held true.
The results were striking. Cell lines predicted by VIPER to have heightened EGFR activity responded positively to the inhibitor—even in cases where standard mutation analysis suggested that the cells were unlikely to be treatable. Conversely, cell lines with low EGFR activity did not show a positive response, despite the presence of EGFR mutations. These findings suggest that VIPER’s method of integrating actual protein activity data can offer a more intuitive and reliable approach to tailoring treatment.
Lessons Learned from the Study
From the lung cancer study, several important lessons emerge that can guide future clinical application of personalized medicine:
- Protein Activity Matters: The study reinforces the idea that focusing solely on gene mutations without considering protein activity is like missing the real engine problem in a car.
- Better Prediction Equals Better Treatment: When oncologists can predict drug response accurately, patients benefit from treatments that are truly effective, reducing exposure to unnecessary side effects.
- Adapting to Complexity: The traditional approach often stumbles when faced with the tricky parts of tumor biology. VIPER’s integration of full transcriptomic analysis is a robust way to work through these complicated pieces.
Such studies highlight the promise of computational techniques in oncology. They also urge the medical community to start thinking about cancer treatment not as a one-size-fits-all solution, but as a tailored approach where every patient’s treatment regimen can be as unique and dynamic as their tumor biology.
Clinical Impact and Future Directions for Personalized Cancer Treatments
The promise of personalized medicine extends far beyond laboratory experiments. With methods like VIPER, clinicians could soon have a super important tool at their fingertips, enabling them to make informed decisions about treatment options based on a tumor’s actual biological behavior. This transition to more precise treatments offers hope not only for better outcomes but also for reduced side effects, as patients would receive treatments targeted precisely at the mechanisms driving their cancer.
Imagine a scenario where a patient’s tumor is biopsied, and its transcriptomic profile is immediately sequenced. That data can then be run through a computational system like VIPER, which figures out the network of active oncoproteins supporting the tumor. With this information, oncologists are better equipped to choose a drug or combination of drugs that directly combat the tumor’s active pathways. This could mean faster recovery times, longer periods of remission, and improved overall patient quality of life.
Future Applications in Clinical Settings
While the current applications of VIPER may appear confined to the realm of academic research, its potential in real-world clinical settings is enormous. Here are several areas where VIPER’s methodology might shine:
- Cancer Screening: By integrating gene expression profiles already gathered during routine diagnostics, VIPER could help identify patients who would benefit from targeted therapies even before the full progression of the disease.
- Treatment Planning: Oncologists could use VIPER to determine the most promising targeted therapy or combinations thereof, thereby personalizing the course of treatment to each patient’s biological needs.
- Monitoring Progress: With repeated measurements over time, VIPER might allow for the tracking of tumor evolution and the timely adjustment of therapies as the tumor’s biology changes.
- Drug Development: Pharmaceutical companies could leverage VIPER insights to design new drugs that specifically target active oncoproteins or to identify biomarkers that predict successful response to treatment.
The transition from bench to bedside is rarely smooth, and there will undoubtedly be nerve-racking challenges along the way. However, the potential benefits of shifting towards a personalized approach in oncology are simply too significant to ignore.
Challenges and Opportunities: Moving Forward with Personalized Oncology
No new approach is without its challenges. Although VIPER shows clear advantages in predicting drug responses by looking at the actual activity of oncoproteins, there are still several intimidating hurdles that researchers and clinicians must overcome before these techniques become standard practice in everyday oncology.
Technical and Logistical Hurdles
One significant challenge is the technical requirement for high-quality transcriptomic data. For VIPER to generate reliable predictions, the biopsy samples must be processed and sequenced with minimal errors. Here are some of the key logistical challenges:
- Data Quality: Ensuring that the transcriptomic data is accurate is super important. Variations in sample collection, handling, and processing can introduce errors that may skew the analysis.
- Computational Resources: Analyzing whole-transcriptome data is computationally intense. Many hospitals and clinical settings may need to invest in robust computational infrastructure to make this feasible.
- Standardization: The medical community will need to agree on standardized methods for data interpretation. Without consistency, translating VIPER predictions into actionable treatment plans across different institutions could be difficult.
These tricky parts, while significant, are not insurmountable. The continuous evolution of bioinformatics tools and the growing accessibility of high-throughput sequencing are key factors that will eventually help steer through these complications.
Opportunities for Future Research and Collaboration
The challenges associated with high-level computational analysis in oncology also open the door for collaboration between multidisciplinary teams. Here are a few exciting opportunities:
- Interdisciplinary Partnerships: Oncologists, bioinformaticians, and data scientists can work together to refine algorithms like VIPER. This collaborative approach is essential for bridging the gap between computational predictions and clinical application.
- Large-Scale Studies: Multi-institutional studies can pave the way toward validating these computational models on a larger patient population. With more data, researchers can fine-tune the approach to cover a broader range of cancer types and subtypes.
- Personalized Data Repositories: Establishing comprehensive databases that collate transcriptomic data from patients around the globe could help build a foundation for even more precise predictions in the future.
- Education and Training: It is essential to train medical practitioners in using advanced computational tools, ensuring that new techniques are both understood and trusted in a clinical setting.
As the field of oncology continues to evolve, the integration of computational methods into clinical decision making is likely to become a cornerstone of personalized medicine.
The Broader Impact of Personalized Oncology on Patient Care
The shift toward personalized cancer treatments has far-reaching implications not only for the scientific community but also for patients and their families. At its core, personalized oncology embodies the hope that treatments can be tuned to the individual, transforming what has historically been an overwhelming battle into a more targeted, manageable fight against cancer.
From a patient’s perspective, the benefits of having treatments tailored to the specific biology of their cancer are profound:
- Better Efficacy: Targeted treatments mean that patients are more likely to receive drugs that have a direct impact on the tumor’s active pathways, potentially leading to quicker and more robust responses.
- Fewer Side Effects: With personalized treatment plans, patients might avoid the side effects that often come with blanket approaches like chemotherapy, as the treatment is designed with a clear target in mind.
- Empowerment Through Knowledge: When oncologists can provide a clear rationale for chosen treatments—backed by data from methods like VIPER—patients may feel more supported and informed throughout their treatment journey.
- Hope for the Future: As personalized oncology evolves, the overall quality of care improves, instilling hope that cancer treatment will continue to advance toward more effective and less intrusive methodologies.
These benefits underscore the importance of continued research and investment in computational approaches to understand the subtle details and little twists of cancer biology. Making your way through the challenges to develop fuller, more reliable models is not just a scientific triumph—it is a patient-centered strategy that could transform lives.
Opinions on the Future Direction: The Promise and Perils of a Personalized Approach
As someone who has observed the evolution of cancer treatment over the years, I find the potential of personalized oncology both exciting and a bit nerve-racking. On the one hand, methods like VIPER hold the promise of demystifying the complicated pieces of tumor biology, allowing clinicians to work through these confusing bits with a clearer understanding of each case’s small distinctions.
On the other hand, the transition to computational-based decision making in a field as sensitive as cancer care comes with important questions and a sense of caution. The following points illustrate a balanced view:
- Optimism About Precision Therapy: There is a genuine excitement among many researchers and clinicians about the breakthroughs personalized treatments can offer. By tailoring therapy based on active protein networks and gene expression, we might finally see higher efficacy rates than those achieved with traditional methods.
- Caution Against Over-Reliance on Technology: It is critical to remember that computational models, no matter how advanced, are tools to assist clinical judgment—not replace it. The art of medicine still requires personal insight, human empathy, and a deep understanding of the patient’s overall health and life circumstances.
- The Need for Continuous Validation: As promising as VIPER is, ongoing research is needed to ensure its predictions stand up to the nerve-racking uncertainties of real-world clinical applications. Long-term studies will be key to embedding these techniques into standard practice.
My personal view is that the promise of personalized oncology is immense. The ability to design a treatment strategy that directly counters a tumor’s active pathways is a game changer. Yet, it is essential to maintain a healthy balance between enthusiasm for technology and the cautious, well-tested protocols that have long governed cancer care.
Integrating Computational Innovation into Everyday Practice
The widespread adoption of computational methods in oncology will require a multifaceted approach that involves not only technological advancement but also changes in how we educate, train, and support healthcare professionals. In the coming years, efforts to integrate VIPER-like systems into routine practice will likely focus on several key areas:
- Clinical Training: Medical schools and professional training programs must incorporate modules that teach new oncologists how to interpret and apply computational data. Understanding the interplay between gene expression and treatment response is crucial for the next generation of cancer specialists.
- Collaboration with Bioinformatics: Hospitals and research institutions will need to create dedicated teams that blend clinical insights with bioinformatics expertise. This collaborative model can help figure a path through the data and turn raw transcriptomic information into actionable treatment plans.
- Regulatory Support: It will be important for regulatory bodies to recognize and establish guidelines for using computational methods in oncology. Clear protocols and standards will not only enhance the reliability of these techniques but also ensure patient safety.
- Patient Engagement: Finally, patients should be informed about the new possibilities of personalized treatment. Transparency about how computational data guides therapy can help build trust between patients and their healthcare providers.
These steps are necessary to make sure that the leap into personalized treatment does not remain a niche practice but becomes a mainstream part of cancer care. In doing so, we honor the strides made in understanding the small distinctions of tumor biology and the responsibility to leverage them for real-world benefit.
Conclusion: A Spotlight on the Future of Cancer Treatment
In conclusion, the emergence of methods like VIPER heralds a significant shift in the ongoing battle against cancer. By looking beyond traditional mutation-based analyses and focusing on the actual activity of oncoproteins, researchers have opened a promising pathway for personalized oncology. This approach addresses the tangled issues and hidden complexities that have long confounded effective treatment selections.
While challenges remain—from ensuring high-quality, consistent data to integrating these advanced techniques into daily clinical practice—the opportunities for improved patient outcomes and more effective drug responses are too substantial to ignore. VIPER not only provides a fresh perspective on what drives tumor growth but also encourages a more thoughtful and direct engagement with the biology of cancer.
The road ahead may be intimidating at times, loaded with both technological and practical twists and turns. However, the potential impact on patient care—offering tailored treatment regimens with fewer side effects and an improved quality of life—is a super important goal that the medical community can rally around.
As we stand at the crossroads of traditional and personalized medicine, it is critical for scientists, clinicians, and policymakers to work in tandem, embracing these new computational tools while maintaining a cautious but progressive outlook. The promise of personalized cancer treatments is real, and with continued research, collaboration, and a balanced integration of technology and human insight, we can look forward to a future in which the fight against cancer is both smarter and more compassionate.
Moving forward, it is essential to remain open to the innovative potential of computational medicine, to celebrate incremental improvements, and to understand that every new piece of data brings us a step closer to a world where cancer treatment is as individual as the patients themselves. By embracing these new insights and techniques, we ensure that the next chapter in oncology is not just about battling a disease, but about understanding and harnessing the unique biological story of every patient.
Originally Post From https://sciworthy.com/toward-personalized-cancer-treatments/
Read more about this topic at
Precision or Personalized Medicine
Precision Oncology | National Institutes of Health (NIH)