Assessing the New Frontier in Prostate Cancer Treatment
Recent early safety data from a phase 2 trial investigating the combination of apalutamide and carotuximab in metastatic castration-resistant prostate cancer (mCRPC) suggest that this therapeutic approach is both promising and manageable. As an editor with a keen eye on modern medicine, I find it essential to take a closer look at this treatment strategy, its potential benefits, and the challenges it might present for patients and clinicians alike. In our exploration today, we’ll dig into the trial’s findings, look at the trial design and its eligibility criteria, and examine how this might change the landscape in mCRPC care.
At first glance, the safety profile of the combined regimen puts forward an encouraging message: no dose-limiting toxicities or unexpected adverse effects have been observed in the initial cohort of patients. Such findings come at a time when clinicians are constantly figuring a path through the tricky parts of developing safe and effective therapies for advanced-stage cancers. It is a period replete with confusing bits and tangled issues that require both caution and innovative thinking. The absence of grade 3 or 4 adverse events in these early results has set the stage for further scrutiny and potential expansion of this approach in larger patient populations.
Understanding Metastatic Castration-Resistant Prostate Cancer
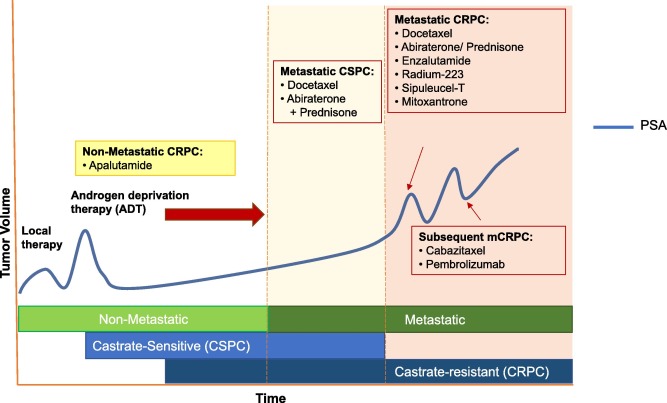
Metastatic castration-resistant prostate cancer is a particularly aggressive form of cancer that arises following prolonged treatment with androgen deprivation therapies and other androgen receptor (AR)-targeted agents. The tricky parts of managing mCRPC are well known—patients often face a series of complicated pieces when their initial treatments begin to fail. Rising prostate-specific antigen (PSA) levels, coupled with progressive disease on imaging studies, point to a condition that is as intimidating as it is challenging.
The limited effective treatment options for mCRPC have driven researchers and pharmaceutical companies alike to dig into potential combinations that might improve outcomes. For many patients, each twist and turn in their treatment journey is fraught with tension, as the progression of the disease can occur despite rigorous clinical management. In light of this, the early trial results showing a favorable safety profile for the apalutamide plus carotuximab combination offer a glimmer of hope. They suggest that there may soon be an additional tool to help patients manage their disease, even as it continues to evolve.
Evaluating the Combination: Carotuximab and Apalutamide
The decision to pair carotuximab—a novel CD105-targeted antibody—with the established AR inhibitor apalutamide reflects a strategic effort to address multiple pathways in cancer progression. This section takes a closer look at each agent, their mechanisms, and how their combination may help overcome the subtle details that have troubled single-agent therapies in the past.
How Apalutamide Works
Apalutamide, known commercially as Erleada, functions by inhibiting the signaling of androgen receptors that are crucial for the growth and survival of prostate cancer cells. The drug’s design has helped it achieve a role in managing mCRPC, even though resistance mechanisms have emerged over time. Patients with a history of CRPC often exhibit rising PSA levels despite therapy with contemporary AR-targeted agents. The key challenge has been overcoming the cancer’s ability to bypass these molecular blockades. With apalutamide already established in clinical practice, its role as a foundation upon which new agents can build further underscores its significance in modern treatment paradigms.
Unpacking the Role of Carotuximab
Carotuximab (ENV-105) targets CD105, a protein involved in tumor angiogenesis—essentially, the process by which tumors develop their own blood supply to support continued growth. By blocking these processes, carotuximab has the potential to interfere with the fuel that tumors require to expand and metastasize. Critically, when combined with apalutamide, there is a hypothesis that this dual approach may not only curb the direct growth signal via the androgen receptor but also limit the tumor’s capacity to sustain its own blood supply.
This dual-target strategy is especially intriguing given the backdrop of advanced prostate cancer, where prior treatments have sometimes managed only to slow the progression of the disease rather than offering a clear-cut benefit in survival. Though more extensive efficacy data are needed, the early phase findings provide an important stepping stone for further clinical exploration.
Digging Into the Phase 2 Trial Design and Patient Eligibility
The phase 2 trial under discussion is methodically designed, with the enrollment of 100 patients over multiple sites across the United States. The trial’s planning highlights a considered approach to both safety and efficacy, balancing exploration with caution. In this section, we break down the eligibility criteria and the trial design to better understand its potential real-world impact.
Eligibility Criteria: Who Stands to Benefit?
Patients who participate in this trial must meet several rigorous inclusion criteria. They are required to:
- Be at least 18 years of age.
- Have a documented history of castration-resistant prostate cancer with rising PSA levels during treatment with a contemporary androgen receptor signaling inhibitor (ARSI) such as abiraterone, enzalutamide, or darolutamide.
- Have received between one and two prior AR-targeted therapies (with apalutamide specifically excluded from previous treatments).
- Either decline taxane therapy or be deemed ineligible for taxane treatment by their treating physician.
Additionally, several exclusion criteria have been put in place to ensure patient safety. For instance, patients with non–PSA-producing prostate cancer, those who have previously received apalutamide or carotuximab, and patients with bleeding disorders or other conditions that present a high bleeding risk are not eligible. This careful vetting is crucial, as it helps clinicians find a way to steer through potential complicating factors and ensure that the patients who enroll in the trial are most likely to benefit from the investigational therapy.
Trial Design: A Two-Pronged Approach
The phase 2 study is built in two parts: an initial safety lead-in followed by a randomized phase. In the safety lead-in stage, the first 10 patients received the combination treatment. Because the absence of adverse effects deemed unacceptable by the trial’s standards allowed the study to proceed, the second stage involves more rigorous testing and random assignment.
Under the randomized design, patients are divided into two treatment arms:
- Monotherapy Arm: Patients receive apalutamide alone at a dose of 240 mg daily, with treatment administered in 28-day cycles. This arm also offers patients the option to cross over to the combination treatment if they experience disease progression.
- Combination Arm: Patients receive the identical oral dose of apalutamide in tandem with intravenous carotuximab, which is delivered according to a specific and carefully structured dosing schedule over multiple cycles.
The dosing schedule for carotuximab is particularly noteworthy. It involves different dose levels during the initial cycle, advancing from 3 mg/kg on day 1 and incrementally increasing to 10 mg/kg and eventually 15 mg/kg by cycle 2 and subsequent cycles. This thoughtful incremental dosing helps ease patients through the treatment regimen while minimizing the potential for toxicity and other complications.
Managing Side Effects: A Closer Look at Adverse Events
While the early safety data indicate that the combination is well tolerated, managing treatment-related adverse events (AEs) remains of key concern. The study has reported that the observed AEs have been manageable with standard supportive care protocols. No grade 3 or 4 AEs have been noted, which, if replicated across a larger cohort, could mark a significant advance in how clinicians tackle setbacks in treatment.
Grading the Adverse Events
In clinical practice, adverse events are graded to help clinicians measure their severity and impact. The lack of high-grade (grade 3/4) events in this study is very encouraging, as it suggests that:
- Patients are likely to experience fewer interruptions in their treatment cycles.
- Standard supportive measures are sufficient to address the side effects that do occur.
- The combination therapy may be a relatively safe addition to the treatment arsenal even for patients with a heavy treatment burden.
It is important to note that while managing side effects is key to improving patients’ quality of life, it is only one side of the coin. Equally critical is ensuring that the treatment delivers tangible improvements in disease control and overall survival. As the trial proceeds, close observation and careful analysis of these dual aspects are essential.
Clinical Implications: How This Study Sheds Light on Future Treatment Options
The early safety data emerging from the combination of apalutamide and carotuximab carries important implications for the future of mCRPC treatment. Many in the oncology community are watching closely, given that mCRPC has long been a tough nut to crack. The results so far suggest a pathway that might offer patients more than just symptom management.
Offering New Hope Amid Challenging Times
For many patients, the journey through castration-resistant prostate cancer is filled with off-putting challenges and nerve-racking decisions regarding escalating treatment options. The possibility of adding carotuximab to an established AR-targeting regimen presents a nuanced opportunity to overcome some of the little details that traditional treatments have failed to address. It is the hope of many experts that this combination could help improve radiographic progression-free survival (rPFS), a measure that is crucial for understanding how long patients might remain free of detectable disease progression.
However, one must also be cautious. While early safety data are promising, it is too early to claim a breakthrough in efficacy or overall survival outcomes. As interim efficacy data are expected in September 2025, discussions around the design of a potential phase 3 study based on these outcomes are already underway. This cautious optimism is a hallmark of thoughtful scientific inquiry—celebrating minor advances while remaining aware that navigating the tricky parts of long-term cancer management involves many twists and turns.
Other Treatments in the mCRPC Landscape
While combination therapies like apalutamide plus carotuximab represent an innovative direction, it is essential to recognize that mCRPC treatment is a multifaceted field. Traditionally, management strategies include:
- Androgen deprivation therapy (ADT)
- Second-generation androgen receptor inhibitors such as enzalutamide and darolutamide
- Chemotherapy with taxanes like docetaxel or cabazitaxel
- Radionuclide therapy such as radium-223 for bone metastases
Each of these approaches is associated with its own set of benefits and challenges, not to mention the inevitable issue of patient tolerance to side effects. The promise of a combination therapy that integrates two mechanisms of action—hormonal regulation and angiogenesis inhibition—could potentially help patients conquer some of the more intimidating aspects of the disease. However, integrating these new pathways into existing regimens will require detailed follow-up, careful statistical analysis, and, most importantly, patient-centered outcomes to truly determine if we have found a breakthrough.
Patient Perspectives: Understanding the Real-World Impact
From the patient’s point of view, the decision to join a clinical trial is no small matter. When grappling with a diagnosis of mCRPC, patients often face a series of layered challenges: physical symptoms, emotional stress, and the sometimes overwhelming burden of coordinating an increasingly complex treatment schedule. These are nerve-racking realities that impact quality of life every day.
The Importance of Informed Decisions
Patients considering participation in such trials need to have a full understanding of the benefits and risks involved. A few key points to consider include:
- Risk vs. Benefit: The initial safety data indicate that many of the side effects can be managed with supportive care. However, every individual will experience treatment differently, and patients must weigh these manageable side effects against the possibility of improved disease control.
- Impact on Quality of Life: While prolonging progression-free survival is a key endpoint in such studies, patients and clinicians alike must consider how the treatment regimen might affect day-to-day well-being. The goal is to deliver therapies that not only extend life but also maintain quality of life.
- Flexibility in Treatment Plans: The design of this trial, with its crossover option for patients in the monotherapy arm who experience progression, acknowledges the need for adaptable treatment plans tailored to the patient’s evolving condition.
Empowering patients with clear, understandable information is essential. Many potential participants often find themselves having to figure a path through a maze of clinical terminologies and statistical measurements. As such, transparent communication from the treating team, along with tailored support and educational resources, becomes a must-have element in modern clinical trials.
Potential Challenges and the Road Ahead
While the data emerging from early-stage trials shine a positive light on the safety of combining apalutamide and carotuximab, several challenges remain. The field of oncology is replete with examples where promising early results did not always translate into long-term clinical success. It is important to remain both optimistic and realistic as we work our way through this evolving scenario.
Addressing the Hidden Complexities
One of the main hurdles in advancing combination therapies is dealing with the hidden complexities that only emerge in larger, more diverse patient populations. Some of these challenges include:
- Long-Term Efficacy: While the initial safety profile is promising, the true test will be whether the combination offers a sustained clinical benefit over the long run. Interim efficacy data, scheduled for release later this year, will help illuminate this question.
- Expanding Patient Populations: Early trials typically involve a smaller, more controlled group of patients. As larger studies are conducted, subtle differences in treatment responses among various demographic and genetic groups may surface.
- Regulatory Hurdles: Discussions with regulatory agencies regarding the design of a potential phase 3 trial will be critical. These regulators will demand robust evidence not only of safety and efficacy but also of the treatment’s ability to offer real improvements in patient outcomes compared to current standards.
Each of these issues requires careful consideration. The oncology community is well aware that every new treatment avenue must be meticulously vetted before being adopted widely. Therefore, while the current data offer a promising look, much work remains to confirm that these early findings can be replicated on a larger scale and over a longer period.
Considering Economic and Accessibility Factors
Beyond clinical safety and efficacy, potential economic and accessibility issues may also come into play as new therapies are introduced. High treatment costs, complex dosing schedules, and logistical challenges related to intravenous administration can create additional layers of twist and turns that need to be elegantly managed. Here are some considerations for the future:
- Insurance Coverage: Will insurance providers readily cover the costs associated with a combined regimen of an established oral medication and a newer intravenous agent? The answer to this remains to be seen.
- Healthcare Infrastructure: The administration of carotuximab requires specialized settings with proper support for intravenous therapy. Expanding this treatment to more patients might necessitate investments in healthcare infrastructure.
- Cost-Benefit Analysis: Ultimately, the healthcare community will need to determine whether the combination offers enough of an advantage over existing therapies to justify the additional costs. This decision will involve both quantitative data and qualitative patient feedback.
As we take a closer look at these factors, it is clear that introducing any new treatment into routine clinical practice is more than just a matter of clinical trial success. The real-world implementation involves a multifaceted approach that must account for economic feasibility, logistical practicalities, and broad access to care. These are all topics that deserve further research and discussion, especially as more efficacy data become available.
A Look in the Crystal Ball: The Future of Combination Therapies in Oncology
The early results from the apalutamide and carotuximab study contribute to an evolving narrative where combination therapies increasingly play a key role in the fight against resistant cancers. As we move forward, several future directions seem evident.
Moving Toward Phase 3 Trials
The next logical step involves expanding the study into a phase 3 trial once additional safety and efficacy data have been collated. Such a trial would aim to definitively assess whether combining these treatments truly offers a statistically and clinically significant advantage over apalutamide alone.
Important aspects that future trials will need to explore include:
- Long-Term Outcomes: Monitoring overall survival, quality of life, and sustained control of disease progression in larger cohorts.
- Subgroup Analyses: Determining if certain patient subgroups—based on genetic markers, previous treatment responses, or demographic factors—derive greater benefit from the combination.
- Refinement of Dosing Schedules: Fine-tuning the dosing regimen to further minimize side effects while maintaining or enhancing therapeutic effectiveness.
Regulatory bodies and industry stakeholders are already starting conversations about the design of the potential phase 3 trials. These discussions are critical, as they will ultimately shape the direction of future research and, possibly, pave the way for a broader implementation of combination therapies in clinical practice.
The Role of Biomarkers and Precision Medicine
A major focus for future research in mCRPC is the use of biomarkers to identify which patients might benefit the most from specific therapeutic combinations. Precision medicine, which tailors treatment to a patient’s individual genetic and molecular profile, is becoming increasingly critical. Key considerations here include:
- Identifying Predictive Markers: Researchers must work to determine which biomarkers can predict favorable responses to the combination of apalutamide and carotuximab. Understanding these markers is key to making your path through treatment options more straightforward.
- Integrating Molecular Profiling: Utilizing advanced genomic and proteomic technologies to guide treatment decisions will allow for a more targeted approach, potentially improving outcomes and reducing unnecessary side effects for patients.
- Continued Research and Collaboration: Collaboration between academic institutions, clinical research organizations, and pharmaceutical companies is essential to advance our understanding of how best to integrate these new treatment modalities into existing care pathways.
The pursuit of precision medicine in this context is not without its own confusing bits and tangled issues. However, as we accumulate more data from trials such as this one, the growing body of evidence will guide clinicians in making informed decisions that best suit individual patient needs. Ultimately, the integration of biomarker-driven therapies represents a promising frontier in oncology treatment, offering a strategy that is tailored, efficient, and hopefully more effective in prolonging meaningful survival.
Expert Opinions: Balancing Optimism with Realism
It is both exciting and nerve-racking to witness the progress of emerging combination therapies from early safety data to potential phase 3 study discussions. As a seasoned observer in the field of oncology research, I am encouraged by the results so far, yet remain cautious about overinterpreting early findings.
There are a few reasons for this cautious optimism:
- Early Data Limitations: While the initial study results are promising, they come from a limited pool of patients. The real test will come when larger, more diverse patient populations are involved.
- Multifaceted Impact: The ultimate success of a new cancer therapy is measured not only in improved progression-free survival but also in overall patient well-being and quality of life over the long term.
- Regulatory and Logistical Hurdles: As discussed earlier, the transition from phase 2 to phase 3 is a critical juncture that will require additional evidence and, potentially, significant adjustments to dosage schedules and patient selection criteria.
Experts in the field have long recognized that while the journey toward innovative treatments is loaded with hurdles, every incremental advance provides valuable lessons that can inform future strategies. The current study, with its encouraging safety data, serves as a building block for future research efforts. It is a step that reminds us of the constant need to adapt and refine our approaches as new data emerge and as research teams work through the frustrating bits and small twists of clinical trial conduct.
Implications for Daily Clinical Practice
If the favorable safety profile of the apalutamide plus carotuximab combination is upheld in larger trials, the implications for daily clinical practice could be far-reaching. Clinicians might soon have an additional, manageable tool in their therapeutic toolkit for treating mCRPC, a disease known for its overwhelming and nerve-racking progression.
Practical Considerations for Clinicians
Here are a few practical points that clinicians might need to consider as this treatment strategy evolves:
- Patient Monitoring: Enhanced protocols for monitoring side effects and ensuring that any adverse effects are promptly managed will be essential. Standard guidelines for supportive care may need to be updated in light of combination therapy regimens.
- Treatment Personalization: Balancing the patient’s overall health status and prior treatment history will be crucial in deciding who is a suitable candidate for the combination therapy.
- Interdisciplinary Collaboration: Effective communication between oncologists, nursing staff, pharmacists, and support teams will be key in ensuring that the treatment is administered safely and efficiently.
This collaborative approach is important, not just for managing the treatment itself, but also for ensuring that patients receive the kind of supportive care that helps maintain their quality of life. In an era when the emphasis is on holistic cancer care, every step taken to improve both safety and efficacy is a step toward a more hopeful future for patients suffering from advanced prostate cancer.
The Role of Patient Education in the Evolving Treatment Landscape
As new treatments emerge and become integrated into clinical practice, educating patients remains a super important priority. The availability of clear, accessible, and detailed information about treatment options can help patients manage expectations and reduce the intimidating aspects of cancer therapy. Educational initiatives should focus on:
- Explaining the Rationale Behind Combination Therapy: Patients benefit from understanding how apalutamide works together with carotuximab to target different aspects of the cancer, potentially leading to better outcomes.
- Clarifying the Treatment Schedule and Monitoring Requirements: Breaking down the complex dosing schedules into digestible pieces helps demystify the process, alleviating some of the nerve-racking uncertainties associated with trial participation and long-term treatment planning.
- Providing Resources on Managing Side Effects: Equipping patients with knowledge about how to manage minor adverse reactions can empower them to take an active role in their treatment journey.
In practice, clinicians and healthcare educators should work together to craft clear messaging and supportive materials that address the subtle parts of treatment planning and the hidden complexities that patients face. This could include workshops, informational brochures, and digital resources tailored specifically for those navigating mCRPC treatment options.
Conclusion: A Promising Step Toward Improved Outcomes
The preliminary findings regarding the use of apalutamide in combination with carotuximab offer an intriguing glimpse into the future of mCRPC therapy. These early safety data, while not yet definitive evidence of enhanced efficacy, mark an important milestone in the journey toward developing more effective combination treatment strategies for a patient population in desperate need of new hope.
The exploration of this dual approach illustrates the spirit of innovation in oncology—finding creative ways to combine existing therapies with novel agents, each addressing different angles of a complicated disease process. The excitement surrounding these findings is tempered by a healthy dose of realism; it is crucial to work through the nerve-racking challenges and small distinctions that arise during the evolution of such therapies.
As we look toward the expected interim efficacy data in September 2025 and further discussions on a potential phase 3 study, it is essential to continue carefully observing how these early promising signs translate into long-term clinical benefit. If validated in subsequent studies, this therapy could provide a much-needed alternative for patients whose treatment options have been increasingly limited over time.
For clinicians, researchers, and patients alike, this combination strategy reinforces the importance of staying informed about new advancements and remaining open to evolving treatment paradigms. As we take a closer look at every twist and turn of the clinical development process, we are reminded that each step—no matter how small—brings us closer to a future where advanced prostate cancer is not only manageable but, ultimately, conquerable.
In summary, the early safety data for the apalutamide plus carotuximab combination in mCRPC patients is a promising beacon of progress. While there are still many layers to unravel and obstacles to overcome, the innovative approach showcased in this trial provides a critical foundation for future research and clinical applications. With robust patient education, careful clinical monitoring, and a rigorous scientific approach, the oncology community can work together to chart a course through the tricky parts and challenging bits that have long defined treatment for mCRPC.
As we continue to build on these initial findings, let us remain both hopeful and vigilant, committed to sorting out the many practical, clinical, and emotional challenges that define modern cancer care. The journey ahead is replete with opportunities to learn, adapt, and ultimately improve patient outcomes—a goal that lies at the heart of every effort in this ever-evolving field of oncology.
Originally Post From https://www.onclive.com/view/early-safety-data-show-tolerability-of-apalutamide-plus-carotuximab-in-metastatic-crpc
Read more about this topic at
Azitra Reports Promising Safety Data from Phase 1b Trial …
Affimed Reports Promising Phase 1 Efficacy and Safety …