Revolutionizing Colorectal Cancer Management with a Tissue-Free MRD Assay
The field of oncology is replete with tricky parts and tangled issues; however, continuous innovations in diagnostics are opening up exciting avenues. One such evolution is the development of a tissue-free molecular residual disease (MRD) assay for colorectal cancer (CRC). This cutting-edge liquid biopsy technology is stirring conversation among professionals because it promises to reduce the confused bits of recurrence detection in cases where traditional tissue samples are unavailable. In this opinion editorial, we take a closer look at the latest study presented at the 2025 European Society for Medical Oncology GI Congress, share our views on its potential implications, and discuss how this new approach could help clinicians find their way through complex treatment decisions.
This article will not only summarize the study’s impressive findings but also serve as an opinion piece that contextualizes the importance of such innovations in everyday clinical practice. We will dive in to examine the performance of this MRD assay, evaluate its role in guiding treatment decisions, and contemplate the broader potential of liquid biopsy in oncology. To make your reading as clear as possible, we have organized the discussion under several subtopics with carefully chosen long-tail keywords that address the unique elements of this study.
Liquid Biopsy in Colorectal Cancer: A Game Changer?
The use of a liquid biopsy has increasingly become a key focal point in modern oncology. With traditional tissue-based testing sometimes proving too nerve-racking or limiting, the tissue-free MRD assay enters the scene as a super important breakthrough. Tissue samples can often be scarce or suboptimal, so this new approach provides an effective alternative that avoids the intimidating hurdles associated with conventional methods.
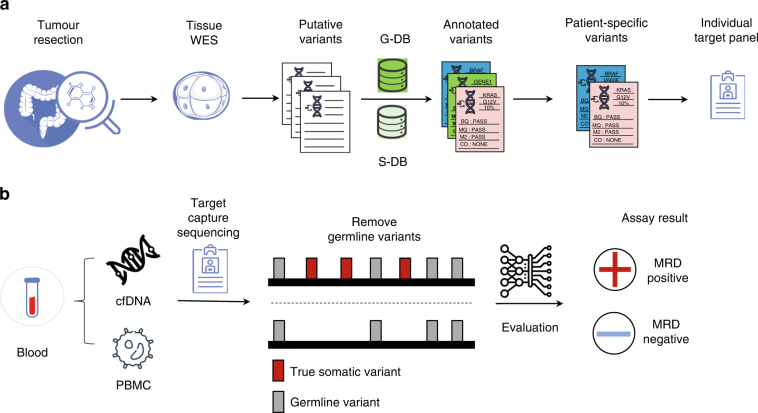
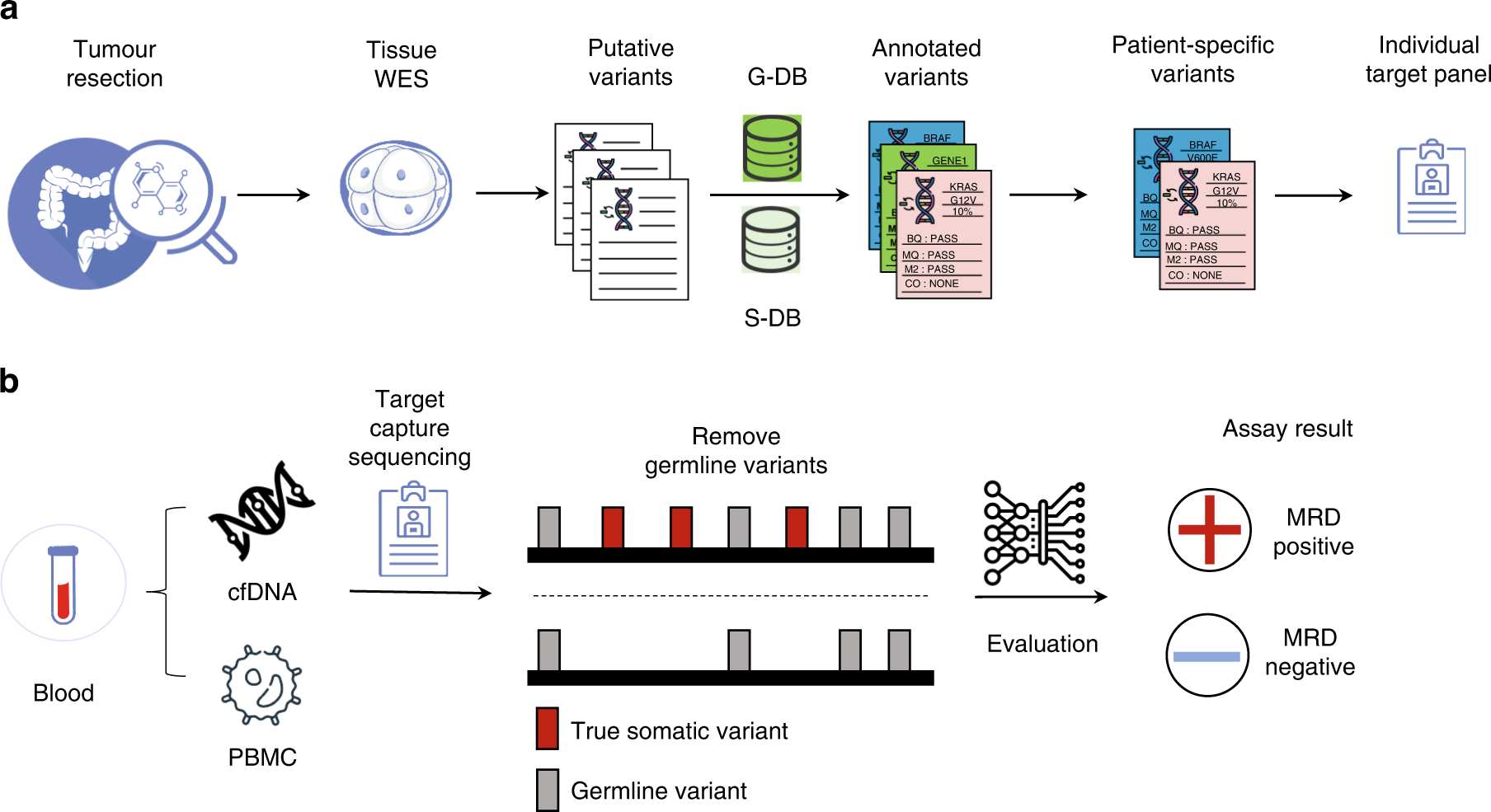
Recent data from the study conducted using the Latitude assay from Natera demonstrated that the liquid biopsy approach delivers excellent overall clinical utility. When applied to approximately 200 patients with resectable CRC, over 1300 plasma samples were tested, which provided a robust picture of the assay’s diagnostic abilities. The ability to get into and analyze circulating tumor DNA (ctDNA) from these plasma samples avoids many of the twisted issues encountered with tissue biopsies.
Key Findings and Their Implications
The study reported several promising statistics indicative of the assay’s powerful capabilities:
- Sensitivity Increase: The assay achieved a sensitivity of 58% during the critical postsurgical MRD window, which improved to 81% in the surveillance setting.
- Diagnostic Lead Time: With a median lead time of 4.6 months for recurrence detection, clinicians are better positioned to identify a relapse way before the conventional methods might signal trouble.
- High Specificity: The tissue-free assay showcased 92% patient-level specificity in the surveillance timeframe and an even more impressive 97% sample-level specificity, minimizing the risk of false positive outcomes.
These outcomes suggest that the test is not only capable of detecting recurrences more effectively but might also serve as a promising tool for predicting how patients will respond to adjuvant chemotherapy. As discussed by experts, this capability to guide therapy decisions based on a patient’s MRD status might help to steer through the complicated pieces of treatment planning.
Molecular Residual Disease Detection in Resectable CRC: Clinical Nuances and Promising Prognostic Value
The detection of molecular residual disease has always been a tricky part in the management of CRC. Traditional methods often fall short, leaving clinicians without a clear picture of which patients might benefit from additional therapy. What makes the tissue-free MRD assay particularly appealing is its dual role in both detecting recurrence and providing prognostic insight. It is a solution that promises to reduce the overwhelming challenges often associated with late recurrence detection.
In the study, patients who tested positive for MRD after surgery, whether in the immediate postsurgical period or during ongoing surveillance, exhibited significantly inferior outcomes. Hazard ratios strikingly underscored this point, with HR values of 10 during the MRD window and 18 during surveillance. These figures bring into focus the super important value of assessing MRD status when trying to figure a path toward more personalized care.
Prognostic Capabilities: Guiding Clinical Decisions
One of the most compelling aspects of the study is how it highlights the assay’s ability to predict recurrence risk. Such forecasting is riddled with tension when based solely on imaging and traditional tumor markers. However, the MRD assay provides a data-backed foundation to make more balanced decisions. Among high-risk stage II and III CRC patients, those who were MRD-positive showed a substantial clinical benefit from adjuvant chemotherapy, while MRD-negative patients did not experience a statistically significant improvement.
In clearer terms, this suggests that by relying on the new tissue-free approach, clinicians can better sort out who might need more intensive treatment or vigilant surveillance, reducing both under-treatment and overtreatment scenarios. It’s all about making sure that therapy is tailored to a patient’s individual needs, a finer point that has the potential to redefine personalized cancer treatment in the coming years.
MRD Assay Benefits in Colorectal Cancer Surveillance: Adding Diagnostic Confidence
Effective surveillance is crucial for early detection of cancer recurrence. In this environment, the tissue-free MRD assay’s high specificity is a game changer. The assay minimizes the necessity for additional, sometimes nerve-racking, diagnostic procedures that can burden both patients and healthcare systems with uncertainty and anxiety.
When it comes to the surveillance setting, the high sample-level specificity, clocks in at 97%, plays a critical role in ensuring that false alarms are kept to a minimum. For patients, this means less exposure to unnecessary follow-up tests and treatments, while for clinicians, it provides a clear signal to get into early intervention protocols. Ultimately, the technology offers a neat package of both detecting disease presence and providing a forecast of recurrence, which builds confidence in decision-making processes.
Comparative Advantages Over Traditional Tissue Biopsy
Let’s juxtapose the benefits of the new tissue-free assay with those of traditional methods. Below is a table that outlines the key differences between liquid biopsy-based MRD detection and conventional tissue biopsies:
| Characteristic | Liquid Biopsy MRD Assay | Tissue Biopsy |
|---|---|---|
| Sensitivity in Recurrence Detection | 81% in surveillance; overall improved early detection | Variable; often lower sensitivity post-surgery |
| Sample Availability | Minimally invasive; continuous monitoring possible | Invasive; dependent on tumor tissue availability |
| Lead Time for Recurrence | Median of 4.6 months early detection benefit | Longer delay in many cases |
| Patient Comfort | Less intimidating, low-risk procedure | More invasive, can be off-putting |
| False Positive Rates | High specificity reduces unnecessary follow-ups | Potential for false results due to sampling errors |
The table reflects that by choosing a liquid biopsy approach, we are not only better equipped to get around the challenging bits of tissue collection but also able to offer patients a path that is less overwhelming and more accurately predictive of their clinical trajectory.
Making the Most of Liquid Biopsy Technologies: Real-World Applications and Considerations
While the clinical data is encouraging, we must also take a closer look at how these cutting-edge techniques can be integrated into everyday practice. It is one thing to observe promising numbers in a study and another to translate these results into a real-world setting where physicians have to work through a maze of small distinctions and subtle details. In practice, the tissue-free MRD assay can greatly simplify the complicated pieces associated with routine monitoring and timely intervention.
Let’s consider several factors that are key for everyday clinical integration:
- Cost Efficiency: With liquid biopsy testing becoming more available, the cost issue, although still challenging, is expected to come down as usage scales up.
- Turnaround Time: The speed of obtaining results plays a super important role in treatment planning; rapid diagnostics leads to better management decisions.
- Technical Expertise: Clinical laboratories and practitioners will need training and adaptation time to incorporate such assays into their workflows.
- Patient Acceptance: Many patients are more comfortable with a blood draw compared to an invasive biopsy, which increases acceptance rates and compliance.
Addressing these factors means that healthcare providers must take a practical approach to integrating this technology while carefully considering its fine points. It is in these operational details that the promise of liquid biopsy will eventually be fully realized, bridging the gap between promising research and everyday practice.
Clinician and Patient Perspectives
The clinical community has always been keen to find methods that simplify the frustrating bits of cancer management while providing informative data. Many clinicians, especially those managing CRC, have remarked on the overwhelming relief that a high specificity MRD assay could offer. For patients, knowing that their recurrence risk can be more reliably assessed with a simple blood test is not just a comfort—it’s a shift towards more personalized and less nerve-racking healthcare.
From the clinician’s point of view, this tissue-free assay offers a dual benefit: it provides early recurrence detection and improved risk stratification which are super important when designing patient-specific treatment plans. Patients benefit from fewer intrusive procedures and an earlier warning system, which is invaluable in a disease where time can be the difference between effective intervention and advanced stage progression.
Personalized Treatment Strategies and the Role of Adjuvant Chemotherapy
One of the most intriguing aspects of the study is its insight into the relationship between MRD status and the benefits of adjuvant chemotherapy. In a subgroup analysis, the assay revealed a significant improvement in outcomes for stage II and stage III CRC patients who were MRD-positive. In contrast, MRD-negative patients did not experience a statistical benefit from additional chemotherapy, prompting a debate about de-escalating therapy when it is unlikely to be advantageous.
These findings pave the way for a more targeted application of adjuvant chemotherapy, ensuring that only those who exhibit clear need receive additional treatment. This tailored approach is especially critical in avoiding the nerve-racking side effects and toxicities linked with unnecessary chemotherapy. By focusing therapy on those who need it the most, the overall healthcare burden is reduced, and patients can enjoy better quality of life without the unwanted burden of overtreatment.
Balancing Treatment Intensity Based on MRD Status
The study data clearly suggests that:
- MRD-Positive Patients: For those who test positive, there is a significant gain from adjuvant chemotherapy, supporting a more aggressive treatment protocol to curb recurrence risks.
- MRD-Negative Patients: Massive overtreatment can be problematic, and patients with a negative MRD result may avoid unnecessary chemotherapy side effects.
This balanced outlook is a shining example of how biomarkers can be used by healthcare providers to make smarter, more personalized treatment decisions. It also highlights how essential it is to tailor treatment not only based on traditional staging criteria but also by incorporating modern molecular insights.
Understanding the Trade-Offs: Sensitivity Versus Specificity in MRD Assessment
Achieving the right balance between sensitivity and specificity is one of the more delicate twists and turns in diagnostic testing in oncology. The tissue-free MRD assay stands out by offering high sensitivity during a critical time window post-surgery and excellent specificity during extended surveillance, effectively addressing two major challenging parts of recurrence monitoring.
While a 58% sensitivity in the immediate postsurgical period might seem moderate, the boost to 81% sensitivity in the surveillance phase is a noteworthy improvement. This shows that, like many modern diagnostics, timing plays a key role in the assay’s overall performance. With high specificity figures of 92% at the patient level and 97% at the sample level in later stages, the results offer concrete reassurance that false positives will be kept at bay.
Trade-Offs in Context: A Comparative Analysis
In order to fully appreciate the balance the MRD assay achieves, we can compare the key parameters side by side:
| Performance Metric | Immediate Postsurgical Window | Surveillance Setting |
|---|---|---|
| Sensitivity | 58% | 81% |
| Patient-Level Specificity | N/A in initial phase | 92% |
| Sample-Level Specificity | N/A in initial phase | 97% |
| Diagnostic Lead Time | Not applicable | Median of 4.6 months |
This side-by-side analysis makes it clear that the assay is designed to perform differently based on the clinical context, maximizing its benefit at the periods when it is needed most. This flexibility is a sign of the technical ingenuity behind the assay, demonstrating its potential to become a cornerstone in CRC management.
Overcoming Clinical Challenges: Implementing Liquid Biopsy as Standard Care
Integrating the tissue-free MRD assay into standard clinical practice is by no means a straightforward task. Like any emerging technology, there are some confusing bits, such as optimizing the timing of sample collection, ensuring robust lab quality controls, and interpreting results in a way that aligns with existing clinical workflows. While the early data is promising, it is loaded with challenges that need careful attention before widespread implementation.
However, the potential benefits far outweigh these obstacles. By embracing a diagnostic tool that can provide clearer insight into disease recurrence and treatment responsiveness, clinicians can reduce the overwhelming number of steps required by conventional approaches. This method gives a reliable early warning system that supports proactive clinical management—a key factor in the overall improvement of CRC patient outcomes.
Steps Toward Standardization and Wider Adoption
The path to standardizing liquid biopsy assays in clinical practice involves several deliberate steps. Here’s a potential roadmap:
- Extensive Clinical Validation: Continued multi-center trials and real-world studies are necessary to verify that the assay performs reliably across diverse patient populations.
- Establishing Protocols: Standard operating procedures should be developed that detail when and how to collect samples, interpret results, and integrate findings into treatment decisions.
- Training and Education: Clinicians and laboratory personnel must receive focused training to get around any confusing bits related to new test interpretation.
- Collaboration: Enhanced collaboration between oncologists, pathologists, and diagnostic companies will be crucial in ironing out the fine points of implementing this technology.
- Insurance and Reimbursement: Securing favorable reimbursement policies will promote broader adoption among healthcare providers.
By tackling each of these steps head-on, the healthcare community can start to peel away the tangled issues that have historically hindered rapid adoption of innovative diagnostic tools. The tissue-free MRD assay, with its tailored sensitivity and specificity, seems poised to become a mainstay in our fight against colorectal cancer.
Liquid Biopsy and the Broader Future of Oncology Diagnostics
The potential of liquid biopsy technologies like the tissue-free MRD assay extends well beyond colorectal cancer. These methods can potentially be adapted to other cancer types, paving the way for a broader revolution in oncology diagnostics. They offer a less invasive, quicker, and more adaptable means of monitoring disease progression and treatment response. In an era where personalized medicine is not just a buzzword but a clinical imperative, such innovations are pivotal.
Liquid biopsy’s role could extend to other solid tumors, offering clinicians consistent, reliable biomarkers in situations where traditional biopsies are either too risky or impractical. With growing research and collaborative efforts, the technique could soon become an essential part of routine cancer management protocols.
Expanding Beyond Colorectal Cancer
There are several emerging areas where liquid biopsy is already showing promise:
- Lung Cancer: ctDNA analysis is rapidly becoming an important tool for detecting resistance mutations and guiding targeted therapies.
- Breast Cancer: Continuous monitoring of residual disease helps in assessing the effectiveness of neoadjuvant treatments and early detection of relapses.
- Prostate Cancer: Liquid biopsies can assist in monitoring treatment response in metastatic cases and provide early alerts for disease progression.
As the field evolves, it’s critical that we continue to invest in and support initiatives that get into the fine shades of these new methods. By doing so, we will not only improve outcomes across a variety of cancer types but also ease the nerve-racking processes often associated with invasive diagnostic procedures.
Opinion: Embracing Innovation Amid Uncertainty
While evidence mounts on the benefits of the tissue-free MRD assay, it is clear that the path forward will require careful implementation. As an observer in this evolving landscape, I find that we stand on the brink of a drastic improvement in how we manage colorectal cancer—and potentially other cancers as well. The early data is not only promising but also encourages us to rethink traditional evaluation methods by giving us the confidence to adopt less invasive yet more effective diagnostic tools.
The journey is not free of challenges. There are still twisted issues related to standardization, testing protocols, reimbursement, and even patient education. Yet, these are challenges that we can overcome by working collaboratively across research institutions, clinical networks, and biotechnology companies.
A Personal Perspective on the Future of Cancer Management
From my viewpoint, the adoption of liquid biopsy represents not just a technical advance but a paradigm shift in our approach toward cancer management. The ability to detect residual disease early, predict treatment outcomes accurately, and tailor therapy based on a patient’s individual molecular profile is a breakthrough that should energize the oncology community.
It is essential, however, to acknowledge that no single assay will completely resolve the challenges of cancer recurrence detection. Instead, it should be viewed as part of a broader toolkit that includes radiological imaging, conventional biomarkers, and, importantly, clinical judgment. In a sense, the tissue-free MRD assay is providing us with a powerful new compass to steer through the nerve-racking uncertainties of post-surgical cancer management.
Conclusion: Charting a New Course in Colorectal Cancer Treatment
In conclusion, the advent of a tissue-free MRD assay for colorectal cancer is a promising step forward in modern oncology. With its high sensitivity, excellent specificity, and ability to provide a critical lead time in recurrence detection, the assay addresses many of the confused bits that have plagued traditional testing methods. By allowing clinicians to figure a path for personalized treatment, particularly when deciding on adjuvant chemotherapy regimens, this innovative approach paves the way for a less intimidating, more accurate, and ultimately more effective cancer management strategy.
Although the implementation of such techniques is not without its tangled issues and technical trade-offs, the potential benefits for both patients and clinicians are too promising to ignore. As we work through the small distinctions and manage our way through the practical hurdles, it is my belief that liquid biopsy will assume a central role in the future of oncology diagnostics.
In an era where timely, personalized, and minimally invasive diagnostics are becoming increasingly critical, innovations like the tissue-free MRD assay offer a beacon of hope. They signal a move toward a future where the intricate balance between sensitivity, specificity, and clinical practicality is finally achieved—making a meaningful difference in the fight against colorectal cancer and reshaping the broader landscape of cancer care.
Final Thoughts: A Call for Continued Innovation and Collaboration
Our journey into the future of cancer diagnostics has just begun. The success of the tissue-free MRD assay should serve as a clarion call for continued innovation and collaboration across the oncology research community. By remaining open to new technologies and working together to overcome the intimidating challenges, we can ensure that every patient receives the most accurate and personalized care possible.
As healthcare providers, policymakers, and researchers, it is our collective responsibility to support these advancements, validate their real-world effectiveness, and ultimately bring them into the mainstream of clinical practice. Only then can we truly make a difference in the lives of those battling colorectal cancer and other malignancies.
It is an exciting time in oncology—a time to celebrate the progress made, while also recognizing that every new discovery brings us one step closer to defeating a disease that has long been a source of uncertainty and worry. Liquid biopsy technologies, with their promise of less invasive and more precise diagnostics, mark a significant milestone in this journey. With further research, careful implementation, and collaboration, there is every reason to believe that the future of cancer management is bright, innovative, and, most importantly, patient-centered.
About the Author
The author is an experienced editor and healthcare specialist with years of experience in modern medicine, alternative treatments, and innovative health technologies. Passionate about driving transformation in patient care, the author continues to advocate for the integration of cutting-edge research into everyday clinical practice to improve outcomes and overall quality of life for patients worldwide.
Originally Post From https://www.targetedonc.com/view/novel-tissue-free-mrd-assay-shows-promise-in-colorectal-cancer-management
Read more about this topic at
Revolutionizing cancer monitoring with carbon-based …
emerging hematocrit-based metrics – a narrative review