Innovative Biomarkers and Their Role in Pancreatic Cancer
The treatment landscape for pancreatic cancer is evolving at an astonishing pace, with new diagnostic biomarkers and device-based therapies helping to untangle the tricky parts of this aggressive disease. Recent advancements have highlighted circulating tumor DNA (ctDNA) as a promising tool for monitoring treatment response in metastatic pancreatic cancer. This approach dives into the small details of the disease, offering oncologists the opportunity to assess progression-free survival in real time, rather than relying solely on traditional imaging techniques.
Studies have shown that ctDNA monitoring can pinpoint the presence of minimal residual disease (MRD) and provide insights into the effectiveness of chemotherapy in patients with unresectable pancreatic cancer. The ARTEMIS-PC trial, for instance, demonstrated that patients who achieved ctDNA clearance had a significantly better objective response rate and disease control rate than those who did not. Many clinicians now see ctDNA not only as a cutting-edge diagnostic tool but as a means to figure a path forward when conventional therapy options seem overwhelming.
While there are still tangled issues concerning the amount of tissue available for next-generation sequencing, the integration of blood-based ctDNA testing helps fill in the gaps. This dual approach of tissue and ctDNA analysis offers a more complete picture of the genetic drivers behind each patient’s cancer, thus allowing for more personalized treatment decisions.
Device-Based Therapies: A Closer Look at Tumor-Treating Fields
Another exciting innovation on the horizon is the use of tumor-treating fields (TTFields), a noninvasive therapy that employs electrical fields to disrupt cancer cell division. TTFields have emerged as a super important piece of the therapy puzzle for locally advanced pancreatic cancer. In the global phase 3 PANOVA-3 trial, the addition of TTFields to standard chemotherapy regimens resulted in observable improvements in overall survival and quality of life.
The trial involved administering a combination of gemcitabine plus nab-paclitaxel, with or without the use of TTFields. Patients who were treated with the TTFields device experienced better overall survival rates and enjoyed prolonged periods without pain and significant side effects. Despite some mild skin irritations associated with the device, the side effects were mostly low grade, thereby making this treatment option a well-tolerated addition to conventional strategies.
The TTFields treatment uses a portable device and strategically placed arrays to deliver electrical fields that interfere with cancerous cell multiplication. By taking a closer look at this technology, healthcare professionals are better able to steer through the challenging aspects of treatment planning in a disease that is notoriously tricky to manage. For many patients, the prospect of a noninvasive approach that complements chemotherapy is a beacon of hope in otherwise nerve-racking treatment scenarios.
Next-Generation RAS Inhibitors and the Challenge of KRAS
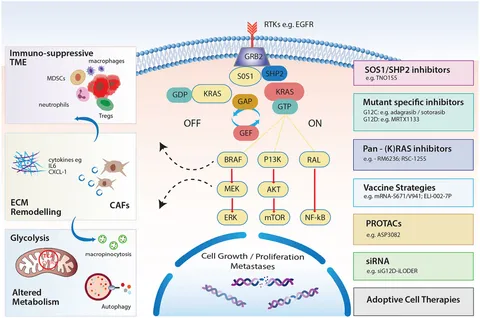
One of the most intimidating challenges in pancreatic cancer has been targeting the KRAS protein, known to drive the aggressiveness of the disease. The stubborn nature of KRAS has historically created many confusing bits when it comes to treatment, largely due to the protein’s role in forming a dense, drug-resistant tumor stroma. Recent therapeutic strides, however, have introduced next-generation RAS inhibitors like daraxonrasib, which have begun to show promising results in overcoming these hurdles.
Early clinical trials involving daraxonrasib indicate encouraging progression-free survival outcomes in patients with KRAS-mutant pancreatic ductal adenocarcinoma (PDAC). These findings have led to breakthrough therapy designations from the FDA for cases that have previously resisted conventional approaches. The process of getting into the fine points of KRAS inhibition is complex, but the emerging data reinforces the critical need for early molecular testing in pancreatic cancer patients.
By regularly incorporating molecular profiling into initial diagnostic evaluations, clinicians may soon be able to match patients to targeted therapies earlier in their treatment journey. This proactive approach not only improves the odds of a favorable outcome but also addresses hidden complexities that have burdened traditional treatment protocols. The development of RAS inhibitors is thus a major leap forward in resolving the tangled issues often encountered in advanced pancreatic cancer care.
New Diagnostic Assays and Personalized mRNA Vaccines in Pancreatic Cancer
Aside from the integration of ctDNA and device-based therapies, there is growing excitement around sophisticated diagnostic assays and personalized mRNA vaccines that aim to detect pancreatic cancer at its earliest stages. Researchers have been working hard to design a rapid, noninvasive assay that uses fluorescently labeled, protease-sensitive peptides coupled with magnetic nanosensors. Known by the acronym PAC-MANN-1, this innovative assay can distinguish pancreatic cancer from other pancreatic diseases with impressive accuracy.
In many ways, this assay tackles the little details of tumor biology, offering a potentially life-saving early detection method that outperforms traditional biomarkers like CA 19-9. For instance, in blind retrospective studies, PAC-MANN-1 achieved up to 98% specificity and demonstrated the ability to detect the disease even in its initial phases. By managing your way through the various diagnostic options available today, oncologists now have a powerful tool for early intervention strategies that could transform patient outcomes.
Complementing diagnostic innovations is the exciting realm of personalized mRNA vaccines. These vaccines are engineered to activate the patient’s immune system by tailoring the vaccine to the unique mutational profile of their tumor. In early-phase trials, mRNA vaccines like autogene cevumeran have shown an acceptable safety profile while sparking an immune response in a significant percentage of patients. Many experts now see these vaccines as a potential game-changer, particularly for patients with advanced disease who have exhausted conventional treatment options.
Technological Advancements and Their Impact on Treatment Planning
Modern treatment strategies for pancreatic cancer are on the move, and the integration of technology into the clinical workflow is making an obvious difference. Oncologists have learned how to use real-time data from ctDNA, advanced sequencing assays, and even device-based therapies to tailor treatment strategies more precisely than ever before. This level of personalized care means that treatment plans can be adjusted quickly in response to the smallest changes in tumor burden or patient health, ensuring that therapy is both adaptive and responsive.
The advent of next-generation technologies has brought about a significant paradigm shift. No longer is treatment solely based on the traditional one-size-fits-all approach; instead, clinicians are now able to make judicious decisions by utilizing a wealth of data available at the molecular level. These efforts help to sort out the complicated pieces of treatment challenges and offer a more comprehensive roadmap for patient care.
- Integration of ctDNA for real-time tracking of disease progression
- Utilization of device-based therapies like TTFields to disrupt cancer cell division
- Targeting KRAS with next-generation inhibitors to overcome therapeutic resistance
- Developing rapid, sensitive diagnostic assays that surpass conventional biomarkers
- Personalized mRNA vaccines as a means to harness the immune system
This multi-pronged approach ensures that each patient’s treatment is built on the most up-to-date scientific insights, skillfully combining the strengths of various therapeutic modalities. It is an encouraging signal that the future of pancreatic cancer treatment may well be on a trajectory toward greater efficacy and improved quality of life for patients.
Trials and Clinical Studies: Shaping the Future of Pancreatic Cancer Therapy
Clinical trials continue to play a pivotal role in determining the best treatment modalities for pancreatic cancer. Large-scale studies such as the PANOVA-3 trial have provided critical insights into the benefits of adding novel therapies like TTFields to existing chemotherapy protocols. In PANOVA-3, not only were overall survival improvements observed, but patients also enjoyed extended durations of pain-free survival. Such outcomes provide hope and enhance confidence in integrating these innovative approaches into everyday clinical practice.
Similarly, trials exploring the role of next-generation RAS inhibitors are fostering a renewed optimism amongst oncologists. With FDA breakthrough designations granted to promising agents such as daraxonrasib, research is fast tracking the development of treatments that address the tangled issues posed by the KRAS protein. The ability to stratify patients based on their molecular profile is a critical step towards crafting super important tailored therapies that meet the nuanced needs of those battling this aggressive cancer.
Parallel studies assessing diagnostic innovations like the PAC-MANN-1 assay further underline the importance of early detection. By getting into the nitty-gritty details of pancreatic tumor biology, researchers are paving the way for clinical protocols that recognize disease sooner and adapt treatment plans before the cancer advances further into more advanced, complicated segments.
Lessons Learned from Integrated Oncology Approaches
One of the key takeaways from emerging research in pancreatic cancer is the undeniable value of an integrated approach. The field has come to appreciate that no single modality will suffice in addressing the expansive, nerve-racking challenges of this disease. Instead, the collective strength of sensitive biomarkers, innovative device therapies, and targeted drug therapies is what promises to overturn the traditionally grim outcomes associated with pancreatic cancer.
Experts in the field emphasize that early molecular testing – combining both blood-based ctDNA analysis and tissue-based next-generation sequencing – is now a crucial component of a comprehensive treatment strategy. This is particularly true given the tricky parts associated with obtaining sufficient tissue samples for traditional analysis. With dual-pronged strategies, oncologists can more accurately track progression and adapt treatment plans, thereby increasing the chances of meaningful clinical benefit and improved overall patient survival.
The collaboration between clinicians, researchers, and technology experts has accelerated the pace at which these advancements move from the laboratory to the clinic, ensuring that treatment remains current with the latest scientific findings. The sharing of data across multicentric trials is streamlining the cumulative knowledge, helping physicians figure a path through the confusing bits and hidden complexities so often encountered in pancreatic cancer treatment decisions.
Implications for Patients and Healthcare Providers
For patients and healthcare providers alike, the rapid evolution in pancreatic cancer management offers both hope and a set of new challenges. On a practical level, patients now have access to pain-free survival strategies and therapies that substantially improve quality of life. For many, the introduction of noninvasive treatments like TTFields means that typical issues associated with more aggressive interventions can be sidestepped.
From the provider’s perspective, the myriad of new data points—from ctDNA levels to molecular sequencing results—has made treatment planning more involved. Physicians must now figure a path that balances the many competing factors, such as patient age, overall health, and specific tumor biology. Despite these intertwined challenges, the potential benefits far outweigh the added complexity. With a higher degree of precision available, the possibility of improving survival outcomes and enhancing life quality has become more tangible than ever.
Moreover, the integration of digital tools, electronic health records, and advanced diagnostic software has empowered clinicians to cope with the subtle details of individual patient profiles. Managing these multifaceted pieces requires multidisciplinary collaboration, as well as a willingness to get into the fine points of each patient’s case. This collaborative spirit is not only fostering innovation but also helping diminish the intimidating aura that has long shrouded pancreatic cancer treatment.
Future Directions: Embracing Personalized Therapy and Technology
Looking to the future, the direction of pancreatic cancer research is clearly oriented towards an even more personalized approach. Upcoming trials such as RASolute-302 and PRISM-1 are currently investigating new therapeutic avenues that build on the insights gained from earlier studies. While the data are still emerging, initial results suggest that the inclusion of CD73 inhibitors and personalized mRNA neo-antigen vaccines could carve out new, promising treatment strategies that go beyond conventional chemotherapy.
These therapies aim to restore immune cell function and modulate the immunosuppressive microenvironment of pancreatic tumors. For instance, quemliclustat, a potent CD73 inhibitor, is currently being evaluated in phase 3 studies with the hope that it will reverse the immune dampening seen in pancreatic cancer. By reawakening the body’s natural defenses, such treatments may work in tandem with targeted agents to not only slow disease progression but also to reverse some of its most overwhelming aspects.
Concurrent to these therapeutic advancements is the promising field of personalized cancer vaccines. Clinical trials have shown that personalized mRNA vaccines, custom-tailored to the specific mutational landscape of a patient’s tumor, can safely stimulate an immune response. Though the development and approval process for such vaccines remains riddled with challenges, the early signals are encouraging. These strategies provide a critical window into the future, where every patient’s treatment is as unique as the genetic makeup of their tumor.
Understanding the Fine Points of Molecular Testing in Pancreatic Cancer
Molecular testing in pancreatic cancer is now considered a super important step in establishing a patient’s treatment blueprint. With the potential to reveal actionable mutations and mechanisms of resistance, these tests allow healthcare providers to customize therapy based on the little twists and turns of the disease’s biology. For example, incorporating tests for KRAS mutations not only guides the use of RAS inhibitors but also sets the stage for participation in clinical trials exploring novel agents.
The process of blood-based next-generation sequencing is becoming a standardized part of clinical practice. Its ability to capture the real-time evolution of the tumor, even when tissue samples are difficult to obtain, represents a major leap forward. By simplifying some of the previously nerve-racking complexities in treatment planning, these tests are proving invaluable in managing patient expectations and outcomes.
This emphasis on early and comprehensive molecular profiling reinforces the necessity for healthcare providers to keep pace with evolving diagnostic technologies. Only by doing so can they provide treatment strategies that are both adaptive and deeply tailored to each patient’s disease. It is a reminder that, in the battle against such an overwhelming enemy as pancreatic cancer, getting into the subtle parts of tumor biology isn’t just academic—it’s absolutely critical.
Integrating Multidisciplinary Approaches for Improved Care
Achieving the best possible outcomes in pancreatic cancer requires more than a single treatment modality; it relies on an integrated, multidisciplinary approach. Surgeons, medical oncologists, radiologists, and pathologists must work together, sharing insights from diverse fields to develop a comprehensive treatment plan. This collaboration is key to managing the tiny challenges and tangled issues that characterize the disease.
In practice, the integration of diverse treatment strategies has led to protocols that combine chemotherapy, targeted agents, and innovative devices like TTFields. Such a comprehensive approach enables the medical team to steer through the maze of treatment decisions, ensuring that every aspect of the patient’s condition is addressed with precision and care. Here are a few bullet points summarizing the benefits of a multidisciplinary strategy:
- Comprehensive molecular profiling for tailored treatments
- Early detection using advanced diagnostic assays
- Noninvasive therapies to reduce side effects and improve quality of life
- Real-time monitoring to quickly adapt treatment plans
- Collaborative decision-making that addresses all aspects of the patient’s health
Such cooperation also helps minimize the intimidating feeling that can arise when faced with a nerve-racking diagnosis. By pooling their expertise, healthcare providers are better equipped to figure a path through the challenging bits of pancreatic cancer care while ensuring that patients receive the highest standard of individualized treatment.
Balancing Innovation and Patient Safety
Despite the substantial promises of these innovative treatments, patient safety remains a key consideration. With every new therapy comes the potential for adverse events. For instance, while TTFields have demonstrated efficacy in improving outcomes, they have also been associated with skin toxicities such as dermatitis and pruritus. Similarly, the introduction of next-generation RAS inhibitors requires careful monitoring for potential side effects that may arise from interfering with essential cellular processes.
Healthcare providers are acutely aware of the need to balance these novel approaches with established safety protocols. Rigorous clinical trials are in place to ensure that any new treatment not only provides a therapeutic benefit but does so without introducing unacceptable risks to the patient. This focus on safety helps build trust among patients and reinforces the importance of combining innovation with caution as we move forward into an era of personalized medicine.
Tables and checklists are often used within clinical settings to monitor patient outcomes and adverse effects. Consider the following table, which summarizes the most common adverse events observed in recent trials:
| Treatment Modality | Common Adverse Events | Management Strategies |
|---|---|---|
| TTFields | Skin irritation, dermatitis, pruritus | Topical treatments, device adjustments, routine monitoring |
| RAS Inhibitors | Nausea, fatigue, laboratory abnormalities | Symptomatic management, dose adjustments |
| mRNA Vaccines | Mild injection site reactions, flu-like symptoms | Observation, supportive care |
The use of such structured tools helps clinicians manage the delicate balance between innovation and safety, ensuring that while new therapies are embraced, the well-being of the patient remains paramount.
Reflections on the Journey Towards Personalized Pancreatic Cancer Care
As we take a closer look at the journey of pancreatic cancer treatment over recent years, it is clear that the integration of advanced diagnostics, novel therapeutic devices, and targeted pharmaceutical agents is reshaping the treatment paradigm. What was once a uniformly bleak landscape is now being transformed by precise, patient-tailored interventions that address every twist and turn of the complex disease process.
Observing these changes compels us to recognize the importance of investing in both research and clinical application. The art of managing pancreatic cancer increasingly requires a willingness to get into the little details that make each patient’s case unique. This commitment to personalized care is not only a testament to the tireless work of scientists and clinicians worldwide but also a reminder that in the face of challenging, nerve-racking diseases, hope is a dynamic and evolving concept.
By embracing a multifaceted approach to treatment, the medical community is working diligently to conquer the confusing bits and tangled issues that have long hindered progress in pancreatic cancer care. The advances we see today are the result of years of persistent study, drawing on lessons learned from both successful therapies and those that fell short. Each step forward is a critical part of a larger journey—a journey that promises to make effective, personalized treatment a reality for patients who once faced an overwhelmingly grim prognosis.
Conclusion: Charting a New Course in Pancreatic Cancer Treatment
The evolving story of pancreatic cancer therapy is a testament to the power of innovation in overcoming the complicated pieces of one of medicine’s toughest challenges. From the promise of ctDNA monitoring and personalized molecular assays to the integration of noninvasive device-based therapies and next-generation RAS inhibitors, each development represents a significant stride toward finer, tailored care.
This new era in oncology is characterized by digital insights, multidisciplinary cooperation, and patient-centered approaches that not only target the disease itself but also enhance the quality of life for those affected. As clinicians and researchers continue to work together to figure a path through the many nerve-racking challenges of pancreatic cancer, the future is gradually becoming less intimidating and more filled with possibility.
In reflection, the success of these innovative therapies depends on early diagnosis, close monitoring, and an integrated treatment plan that considers every subtle detail of the patient’s condition. As we forge ahead in this rapidly changing landscape, the importance of early molecular testing and personalized medicine cannot be overstated. It is a continuous cycle of learning, adapting, and improving—a cycle that brings us ever closer to the day when pancreatic cancer can be managed more effectively, giving patients a better chance at longer, healthier lives.
For healthcare providers, staying updated with these advances means not only adopting new technologies but also being proactive in referring patients to ongoing clinical trials. These trials are on edge with promise and offer opportunities for patients to access the latest, most advanced treatments available. In this way, each new piece of research builds a more robust foundation for personalized care, helping to steer through the myriad challenges posed by pancreatic cancer.
Ultimately, the efforts we see unfolding in this field highlight that the future of pancreatic cancer care rests on the marriage of precision diagnostics, innovative treatment modalities, and an unwavering commitment to patient safety. As we continue on this journey, every new study, every trial, and every technological breakthrough lights the way forward—reminding us that even the most twisted issues can be unraveled with a combination of research, technology, and compassionate care.
Originally Post From https://www.targetedonc.com/view/precision-oncology-spans-biomarkers-and-device-therapy-targeting-kras-in-pancreatic-cancer
Read more about this topic at
Recent advances in precision medicine for pancreatic …
Advances Move Precision Oncology Forward in Pancreatic …