Revolutionizing Lung Cancer Treatment: A Dual-Target Approach on the Horizon
The battle against lung cancer remains one of the most intimidating challenges in modern healthcare. As the leading cause of cancer-related death in the United States—claiming more lives than colon, breast, or prostate cancers combined—it is essential that we figure a path through the tangled issues of treatment resistance. Recent research from the University of Missouri’s School of Medicine is opening promising new avenues, focusing on a dual-target strategy that exploits a molecular “seesaw” within cancer cells. In this opinion editorial, we take a closer look at this breakthrough, discuss the potential impact on treatment paradigms, and explore how these findings could shape the future of lung cancer management.
Understanding the Molecular Seesaw in Lung Cancer
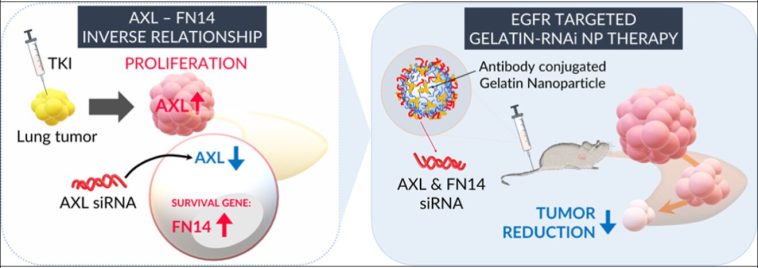
Scientists have long observed that many patients initially respond well to lung cancer treatments, only to see the tumors grow back after approximately 18 months. This puzzling phenomenon has been a subject of intense research and debate. The research team, led by Dhananjay Suresh, Anandhi Upendran, and Raghuraman Kannan, discovered that two proteins—AXL and FN14—function as a molecular seesaw within the tumor cells. These proteins operate in such a way that blocking one seems to trigger the other, which in turn fuels the tumor’s growth and drug resistance.
Innovative Lung Cancer Nanoparticle Treatments
The idea of using nanoparticles for targeted drug delivery has been gathering steam over the past few years, and this latest research marks a significant leap forward. Instead of focusing solely on one protein, the researchers devised a strategy that uses a gelatin-based nanoparticle to shut down both AXL and FN14 at the same time. This dual strategy is critical because it tackles the tricky parts of how tumors can adapt. By stopping both proteins from compensating for each other, the treatment promises to keep drugs effective for a longer period.
Molecular Seesaw in Cancer Drug Resistance Explained
The hidden molecular seesaw discovered by the team underscores one of the confusing bits of cancer drug resistance. Initially, treatments that only blocked AXL were met with disappointing results, as tumors found a detour by upregulating FN14 and continuing to grow. This discovery highlights that cancer cells are not static—they are dangerously adept at adjusting their internal machinery to survive therapy.
Dual-Target Approach: Tackling Tumor Survival from Two Fronts
The clinical implications of using a dual-target approach are vast. Targeting both AXL and FN14 simultaneously addresses the twisted turns of tumor adaptation. Kannan, a professor and chair in cancer research, emphasizes that the dual-target strategy essentially stops both sides of the seesaw from moving. This could potentially transform lung cancer treatment from a short-lived intervention into a long-term management strategy, turning what is currently an off-putting race against time into a more manageable process.
The Role of Nanoparticle Delivery Systems in Modern Medicine
Nanoparticle drug delivery systems have gained attention because they help steer through the little details involved in transporting therapeutic compounds directly to the tumor site. The use of a gelatin-based nanoparticle in this study not only improves drug concentration at the tumor location but also minimizes side effects that are common with systemic therapies. In early studies using mice, the tumors exhibited a favorable response to this dual-target treatment, suggesting that these delivery systems might be key to overcoming some of the complicated pieces of conventional treatment resistance.
Precision in Drug Delivery: How Nanoparticles Make a Difference
Nanoparticles are designed to carry drugs and release them in a controlled manner at the exact location where they are needed most. This targeted approach maximizes drug efficiency while minimizing collateral damage to healthy tissues. In the context of lung cancer, where the tumors can be especially aggressive, using such finely tuned systems is not just a scientific novelty—it is a crucial improvement over traditional treatment methods.
Benefits of Gelatin-Based Systems in Cancer Therapy
Gelatin-based nanoparticles, in particular, offer several attractive advantages:
- They are biodegradable and biocompatible, which means they break down into non-toxic components in the body.
- Their natural origin reduces the risk of adverse reactions when compared to synthetic alternatives.
- They provide an optimal matrix for delivering multiple agents concurrently, making them ideal for dual-target treatments like the one in discussion.
Challenges in Lung Cancer Drug Resistance
One of the most nerve-racking aspects of lung cancer treatment is tumor adaptation. When patients begin therapy with tyrosine kinase inhibitors—drugs designed to specifically target mutated genes—patients often see promising results at first. Over time, however, tumors evolve mechanisms to resist the drugs, resulting in aggressive regrowth. This phenomenon is not unique to lung cancer but is particularly poignant in this disease, where the stakes are incredibly high.
Understanding the Hidden Complexities of Drug Resistance
Resistance to treatment occurs due to several factors. Some of the key contributors include:
- Mutational changes: Tumors can mutate further, rendering drugs less effective.
- Alternative signaling pathways: When one pathway is blocked, cancer cells might rely on another to continue growing.
- Microenvironmental factors: The surrounding tissues can influence how drugs function and are metabolized.
These factors all contribute to a situation that is full of problems—where singular targeting may not be enough. The dual-target approach looks promising because it abandons the one-size-fits-all mindset and adapts to the fighting spirit of the tumor.
The Impact of a Dual-Strategy on Overcoming Treatment Resistance
Dr. Kannan’s team’s novel approach acknowledges that blocking only one protein—like AXL—is akin to playing whack-a-mole with cancer: as you suppress one pathway, another emerges, fueled by FN14. This seesaw effect is a stark reminder that cancer is dynamic and demands a dynamic response. By moving forward with a strategy that simultaneously targets both proteins, researchers hope to reduce the chances that the tumor will find an alternate route to survival. This dual-protein strategy is a significant step towards making lung cancer a more manageable, chronic condition rather than an immediately life-threatening one.
Future Prospects: Advancing Personalized Lung Cancer Therapies
The promise seen in laboratory studies with mice offers a beacon of hope. However, transitioning from preclinical results to widespread clinical application involves considerable work. Future research will need to explore several key questions:
- Does the molecular seesaw effect occur in other types of proteins besides AXL and FN14?
- How will these nanoparticle-based treatments perform in human clinical trials?
- What are the long-term implications of dual-target treatments on patient survival and quality of life?
Personalized Medicine: Tailoring Treatments to Individual Tumor Profiles
Each lung cancer patient’s tumor comes with its own set of fine points—subtle details that demand a customized treatment approach. The study under discussion is a step toward more personalized medicine, where the therapeutic strategy is adapted to the specific molecular makeup of an individual’s cancer. This individualized approach could dramatically improve outcomes, as treatments are tailored to counteract the tumor’s particular mechanisms for drug resistance.
Bridging the Gap Between Lab Research and Clinical Application
While the preclinical results are promising, the journey from the lab bench to the bedside is often long and loaded with issues. Several regulatory, logistical, and financial challenges need to be sorted out before dual-target nanoparticle therapies can become a standard part of lung cancer treatment protocols. Nonetheless, the research team is optimistic, encouraging further investigations to fine-tune the strategy and eventually usher in a new era of lung cancer management.
Comparative Insights: Traditional vs. Dual-Target Therapeutic Approaches
The conventional approach to lung cancer treatment often relies on singular targeted therapy—focusing on one pathway or protein at a time. This method has its merits but has repeatedly shown limitations, especially when cancer cells adapt over time. The dual-target strategy, as highlighted in the recent study, offers several advantages:
- Enhanced Efficacy: By addressing two critical proteins that help the tumor survive, the treatment can potentially maintain its effectiveness much longer.
- Reduced Likelihood of Resistance: The tumor’s ability to shift between proteins (like a seesaw) is curtailed, which may lead to longer-lasting responses.
- Potential for Lower Dosages: Targeted delivery via nanoparticles may allow for reduced dosages, thereby minimizing side effects that are common with higher doses of systemic therapies.
Advantages of Dual-Protein Inhibition
Dual-protein inhibition isn’t just about attacking the tumor on two fronts; it’s about disrupting the very survival mechanisms of the cancer cell. In addition to improved efficacy and reduced drug resistance, this method offers a more resilient framework against tumor adaptability. As researchers continue to poke around the fine details of cancer biology, the dual-target approach may well pave the way for therapies that are not only more effective but also capable of turning lung cancer into a manageable condition for many patients.
Lessons Learned from Traditional Therapies
Traditional therapies have taught us that the road to conquering lung cancer is full of twists and turns. Early treatments showed that while initial drug responses could be dramatic, over time the tumors would tweak their internal systems to elude therapy. This dual-target research underscores the need for therapeutic approaches that are robust enough to counteract those rapid adjustments. It’s a reminder that in the fight against cancer, a flexible and multi-pronged strategy is crucial.
Broader Implications for Cancer Research and Healthcare Innovation
The implications of this research stretch far beyond lung cancer. The concept of a molecular seesaw may be relevant to other forms of cancer as well. Many tumors in different parts of the body exhibit similar survival mechanisms, whereby blocking one cellular pathway inadvertently activates another. Recognizing this pattern could redefine our approach to many cancer types, leading to breakthroughs in how we manage drug resistance more broadly.
Translational Research and Its Role in Healthcare
Translational research, which aims to shorten the gap between lab discoveries and real-world treatments, is super important in the context of these findings. By taking the lessons learned from lung cancer studies and applying them to other cancers, scientists hope to establish a framework for dealing with drug resistance more effectively. This approach not only fosters innovation but also builds a stronger, more resilient healthcare system capable of adapting to emerging challenges.
Interdisciplinary Collaboration: A Key Ingredient for Success
One of the most encouraging aspects of this research is the collaboration between experts in various fields. The team at the University of Missouri, which includes professionals from the School of Medicine and the College of Engineering, exemplifies how working through the tangled issues of complex healthcare problems requires input from diverse disciplines. This interdisciplinary approach allows for fresh, innovative solutions that might not emerge when experts work in isolation.
Expert Opinions: What Healthcare Professionals Are Saying
Leaders in oncology and cancer research across the globe have reacted positively to the study, noting that it represents a bold step in the right direction. Many experts agree that the dual-target strategy is an exciting development because it directly addresses the little twists and subtle parts of drug resistance that have long stumped researchers.
Perspectives from Oncologists and Medical Researchers
Several oncologists have highlighted the following points regarding this breakthrough:
- Enhanced Therapeutic Window: By fine-tuning the delivery of drugs through nanoparticles, the treatment may offer a safer profile with fewer side effects.
- Long-Term Efficacy: The dual approach might extend the period during which patients benefit from targeted therapies, leading to better long-term management of lung cancer.
- Potential for Combination Treatments: Experts point out that this strategy could potentially be combined with other therapies, such as immunotherapy, to create a multi-layered defense against the tumor.
Patients’ Hopes and Future Outlook
For patients, the prospect of a treatment that not only shrinks tumors initially but maintains its effectiveness over time provides fresh hope. While the journey towards clinical application is still in its early stages, the dual-target approach suggests that lung cancer could eventually become a chronic condition that patients manage rather than a terminal diagnosis.
Potential Transformative Impact on Lung Cancer Treatment Paradigms
Traditional paradigms of lung cancer treatment are being rethought in light of these new findings. The dual-target nanoparticle approach represents a significant paradigm shift—one that acknowledges the need to address the small distinctions in how cancer cells adapt and survive. By effectively shutting down both ends of the molecular seesaw, this method could lead to more stable and enduring responses in patients who have historically seen cancer regain ground after initial treatment success.
Integrating Novel Therapies into Current Treatment Strategies
Integrating this dual-target approach with existing treatment regimens requires a thoughtful and systematic strategy. Here are some of the key steps and challenges clinicians might face:
- Rigorous Clinical Trials: Extensive studies will be necessary to test the safety and efficacy of these nanoparticles in humans. Early promising results in animal models need to be validated through multiple phases of trials, ensuring that the treatment not only works but also has an acceptable safety profile.
- Combination Therapy Protocols: Researchers and clinicians will need to determine whether the dual-target strategy can be effectively integrated with other therapeutic methods such as chemotherapy, radiation, or immunotherapy. Synergistic effects could lead to even better outcomes, but proper dosing and scheduling remain a challenge.
- Tailored Treatment Regimes: As our understanding of a patient’s tumor evolves, personalized treatment regimes that factor in the unique characteristics of the cancer become necessary. The development of robust biomarkers to track protein activity like that of AXL and FN14 will be key in this integration process.
Regulatory and Ethical Considerations
With every new medical breakthrough come regulatory and ethical challenges. The journey from promising laboratory data to approved clinical therapy is rigorous and full of twists and turns. Regulatory bodies will demand clear evidence of both efficacy and safety. At the same time, ethical considerations, particularly around patient consent and the management of expectations, must be handled with care. Given the stakes, transparency in clinical trials and open communication with patients will be essential.
Looking Ahead: The Future of Lung Cancer Research
The study from the University of Missouri signals a turning point in lung cancer research. By focusing on the dual inhibition of AXL and FN14, scientists are making significant headway into understanding how tumors evolve to resist treatment—a problem that has long been on edge in the field of oncology. If further research and clinical trials confirm the early promising results, we might be on the brink of a major transformation in how lung cancer is treated.
Continuing the Journey to Discover Hidden Complexities
One of the most exciting aspects of this progression is the potential to uncover similar molecular seesaw effects in other cancers. Researchers are hopeful that the lessons learned from this dual-target nanoparticle approach can be applied beyond lung cancer to tackle stubborn drug resistance in other tumor types. As we continue to dig into the fine points of cancer biology, each discovery helps build a clearer road map for developing even more effective therapies.
Future Investigations: Expanding the Dual-Target Concept
Future studies will likely explore whether the dual-target approach can be expanded to include other proteins or molecular pathways involved in tumor survival. The questions that remain include:
- Does blocking additional proteins further reduce the possibility of resistance?
- How can nanoparticle technology be optimized for even more precise drug delivery?
- What combination of therapies will yield the best results for different patient demographics?
Addressing these inquiries will not only help refine lung cancer therapy but may also revolutionize treatment approaches for a variety of other cancers. As the research evolves, the potential for turning cancer into a controllable, chronic condition becomes more tangible.
Conclusion: A New Path Forward in the Fight Against Lung Cancer
The research led by experts at the University of Missouri offers an exciting glimpse into the future of lung cancer treatment. By targeting both AXL and FN14 simultaneously with innovative, gelatin-based nanoparticles, scientists are addressing the tricky parts of tumor adaptability in a way that could finally keep therapies effective for longer periods. Although these findings are yet to make the leap into everyday clinical practice, they provide a robust foundation on which future, more personalized treatments might be built.
This dual-target approach is a promising new chapter in the fight against lung cancer. The research tackles the dangerous twists and turns of drug resistance head-on, offering both patients and healthcare professionals fresh hope. As we continue to figure a path through the challenging landscape of cancer treatment, it is clear that a multi-disciplinary approach—combining cutting-edge technology, insightful biological research, and collaborative clinical investigation—will be critical to turning lung cancer from an overwhelming threat into a manageable disease.
As we step into an era where personalized medicine is becoming a key player in cancer therapy, innovations like these stand at the forefront of change. They remind us that each carefully studied protein, each nanoparticle engineered with purpose, and each clinical trial undertaken, brings us one step closer to a day when lung cancer is no longer a ticking time bomb but a condition that patients can live with—thanks to treatments that are as dynamic and adaptable as the diseases they aim to conquer.
In summary, researchers at the University of Missouri are pioneering a dual-target strategy that holds the promise of extending the duration of effective lung cancer treatment. By dancing with the dangerous duality of the molecular seesaw and harnessing the precision of nanoparticle delivery systems, they are laying the groundwork for a future where lung cancer may become a chronic, manageable condition rather than an immediate death sentence. The road ahead will undoubtedly be loaded with problems and nerve-racking challenges, but every step taken in innovation sows seeds of hope among patients, clinicians, and the entire field of oncology.
As we move forward, we must continue to support interdisciplinary research and maintain a rigorous commitment to exploring every little twist in the molecular mechanisms of cancer. Only by doing so can we ensure that breakthroughs like these eventually translate into tangible improvements in the lives of those battling lung cancer. With each new discovery, the narrative is rewritten, turning overwhelming odds into stepping stones for a brighter, healthier future.
The journey towards overcoming lung cancer is far from over, but the insights provided by this dual-target research are both refreshing and transformative. They not only challenge us to rethink how we approach cancer treatment but also empower us to keep pushing the boundaries of modern medicine. In the end, the success of such innovative treatments will depend on our ability to embrace both the promise and the pitfalls of new scientific paradigms, ensuring that progress in research always translates into renewed hope for patients worldwide.
Originally Post From https://www.futurity.org/why-lung-cancer-treatments-stop-working-3286112/
Read more about this topic at
mechanisms of dual protein targeting in eukaryotes – PMC
Protein folding as a driving force for dual protein targeting …