Rethinking CAR-T Therapy: The Role of T Cell Characteristics in Hematological Malignancies
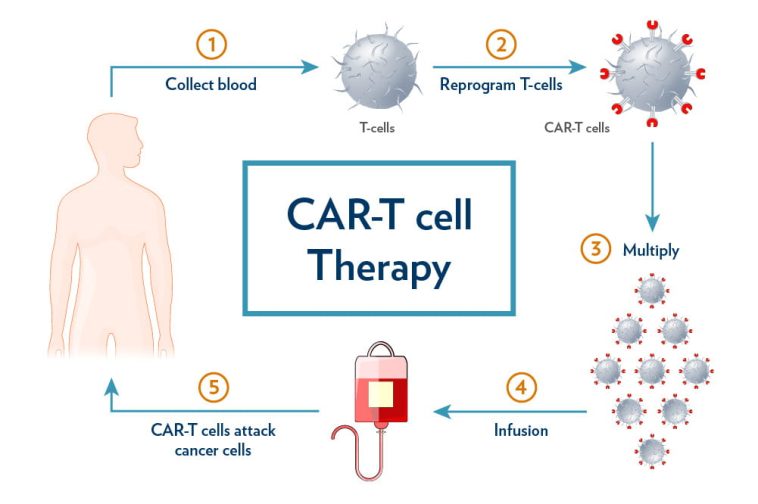
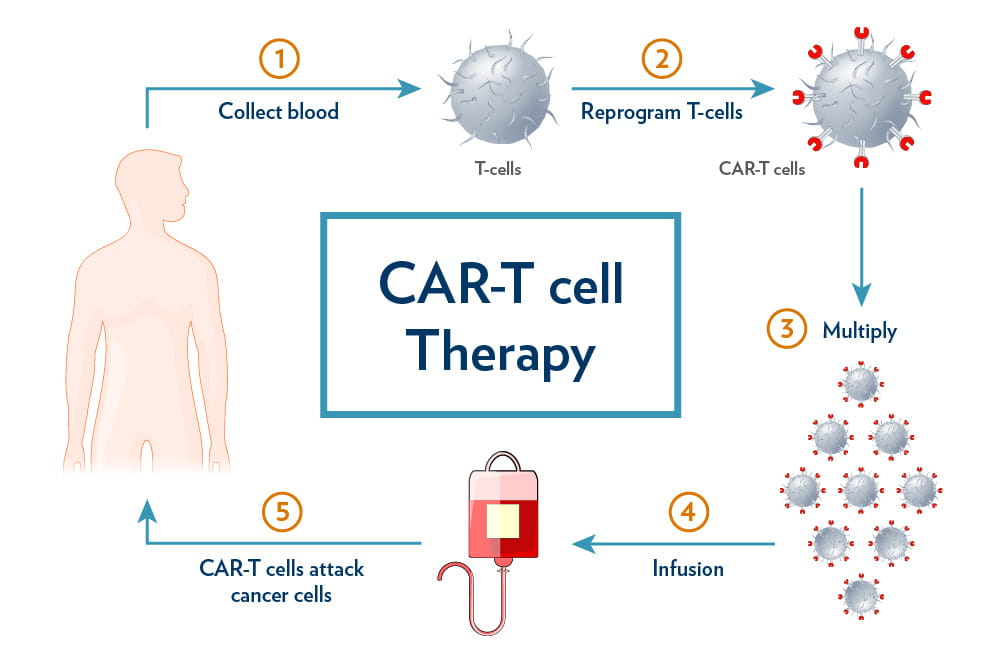
The field of CAR-T cell therapy has sparked a revolutionary change in cancer treatment, especially when it comes to hematological malignancies such as B-cell leukemias and lymphomas. However, success is not always guaranteed. With relapse rates in some B-cell cancers soaring over 50%, researchers continue to explore the tangled issues behind these outcomes. In a recent comprehensive review by experts from University Hospital Ostrava and the Faculty of Medicine at the University of Ostrava in the Czech Republic, we see a detailed analysis of how inherent T cell traits—such as exhaustion, memory differentiation, senescence, regulatory activities, metabolism, and T-cell receptor (TCR) diversity—shape the efficacy of CAR-T therapies.
This editorial aims to take a closer look at the core factors influencing CAR-T cell performance. By examining the subtle details behind T cell dysfunction, exploring metabolic constraints, and assessing the importance of TCR diversity as a potential biomarker for patient response, we hope to provide insights into better therapy design. Let’s dig into the subject matter and evaluate the critical, yet tricky parts of T cell biology, which can turn therapeutic promise into a real-life lifesaver—or, unfortunately, into a therapy with unpredictable outcomes.
Understanding T Cell Exhaustion and Its Impact on Therapy
One of the most discussed topics in this arena is T cell exhaustion. Over time, repeated stimulation—even in the context of life-saving therapies—can lead T cells to lose their capacity to proliferate and destroy tumor cells. This exhaustion is a key piece in explaining why even breakthrough CAR-T therapies sometimes falter. Patients with exhausted T cells often experience limited tumor control, leaving the door open for relapse and progression.
How T Cell Exhaustion Develops
T cell exhaustion can be thought of as a gradual process where the T cells become overworked. Much like any worker facing nerve-racking demands over a long period, T cells eventually lose their sharp edge. Factors contributing to exhaustion include:
- Prolonged antigen exposure
- Repeated cycles of cell stimulation
- Unfavorable cytokine environments
In many cases, there is sufficient evidence to suggest that interventions like PD-1 blockade or even the use of new gene-editing techniques (such as CRISPR) could rejuvenate these tired cells. By limiting the extent of ex vivo expansion, which also plays a role in this issue, clinicians may find that boosting T-cell fitness improves overall responses to treatment.
Strategies to Overcome Exhaustion
To figure a path through the problem of T cell exhaustion, several strategies are under investigation:
- PD-1 Blockade: Using checkpoint inhibitors to prevent the signaling pathways that lead to exhausted states.
- CRISPR Editing: Directly modifying the genetic blueprint of CAR-T cells to enhance performance and longevity.
- Optimizing Ex Vivo Expansion: Reducing the time cells spend outside the body to minimize exhaustion.
By tackling these confusing bits of T cell dysfunction early on, we can create CAR-T therapies that have improved persistence and better tumor control. This approach is more than just a tweak; it’s a rethinking of how to harness the body’s natural defenses in a way that is both efficient and sustainable.
Memory Differentiation: The Value of T Cell Persistence
A recurring theme in recent studies is the significance of early memory T cells. Early memory subsets, such as TSCM (stem cell memory) and TCM (central memory) cells, are considered super important because of their enhanced ability to persist and expand over time compared to their highly differentiated effector counterparts.
Why Early Memory T Cells Are Essential
Memory T cells are the body’s original survivors, capable of adapting and mounting responses even after long periods of dormancy. Their ability to stay poised for action makes them ideal candidates for producing long-term remissions. In the context of CAR-T cell manufacturing, prioritizing these cells may ensure that therapies remain robust even when facing the tricky parts of aggressive hematological malignancies.
The Fine Points of Memory Differentiation
The distinction between different memory T cell subsets is subtle yet significant. Early memory cells carry a cocktail of advantages:
- Greater Expansion Capacity: They can multiply more efficiently after re-encountering antigens.
- Improved Persistence: They have a longer lifespan within the patient’s body, maintaining surveillance over cancer cells.
- Enhanced Anti-Tumor Activity: Given their readiness to respond, they contribute to better tumor control.
These characteristics underline the need for a refined approach in the selection process of T cells for CAR-T manufacturing. The debate now centers around how best to isolate and preserve these early memory T cells while also addressing the limitations posed by prior chemotherapy or inherent immune deficiencies.
Tackling Senescence and the Decline of T Cell Function
Senescence in T cells refers to the phenomenon where cells enter a state of irreversible growth arrest. Senescent T cells exhibit limited proliferative capacity and diminished cytotoxic abilities. These off-putting attributes compromise the effectiveness of CAR-T therapies, creating a critical bottleneck in treating relapsed or refractory disease.
Understanding the Causes of T Cell Senescence
Several factors contribute to the onset of T cell senescence. For many patients, the senescence of T cells is driven by:
- Aging: As patients age, their cells naturally undergo changes that reduce overall function.
- Prior Chemotherapy: Repeated cycles of cytotoxic treatments can push T cells over the edge.
- Inherent Immune Deficiencies: Some individuals are born with immune systems predisposed to early senescence.
If senescence is left unchecked, even the most promising therapies may fall short, as the T cells simply do not have the requisite energy to fight off cancer. The challenge lies in identifying these senescent populations early and finding ways to rejuvenate or bypass them.
Potential Solutions to Overcome Senescence
Several solutions are being explored to manage this nerve-racking issue:
- Telomerase Activation: Activating enzymes that restore the length of telomeres to extend the life of T cells.
- Senolytic Agents: Using drugs designed to clear senescent cells and make room for healthier, more active cells.
- Pre-Treatment Optimization: Modifying the patient’s treatment regimen to minimize damage to T cells before CAR-T infusion.
By addressing the tangled issues of T cell senescence, researchers hope to unlock new avenues for enhancing the overall durability and effectiveness of immunotherapy in hematological malignancies.
Regulatory T Cells (TREG) and Their Dual Role
Regulatory T cells, or TREG cells, are essential for maintaining immune system balance, but their role in CAR-T cell therapy is a double-edged sword. While these cells are critical for preventing autoimmune responses, an overabundance of TREG cells within the CAR-T product or the patient’s body can stifle the desired anti-tumor activity.
The Tightrope of TREG Influence
TREG cells may dampen the immune attack on cancer cells by exerting an immunosuppressive effect. On one hand, they are vital for keeping the immune system under control; on the other hand, they may hinder the therapeutic potential of CAR-T cells. The fine balance of TREG cells is one of the small distinctions that can tilt the scales between a successful response and a relapse.
Strategies for Mitigating TREG-Related Immunosuppression
To tackle this loaded with issues scenario, researchers are exploring several approaches:
- Selective Inhibition: Designing molecules that can selectively modulate TREG activity without compromising overall immune function.
- Targeted Depletion: Using immunomodulatory agents to temporarily reduce TREG populations during critical phases of CAR-T therapy.
- Engineering CAR-T Cells: Modifying the cells to resist the suppressive effects of TREG cells.
By getting into the nitty-gritty of TREG dynamics, scientists hope to strike the right balance—ensuring that the immune system’s brakes do not inadvertently stall the therapeutic engine driving tumor eradication.
Metabolic Challenges and T Cell Fitness
Metabolic activity within T cells is a key step in their functionality. This includes how they produce and utilize energy—a creamy blend of oxidative phosphorylation and glycolysis that supports their rapid growth and sustained anti-tumor activity. However, when metabolic constraints come into play, CAR-T cells can become less effective at sustaining long-term responses.
Understanding Metabolic Constraints in T Cells
The metabolism of T cells is one of the most critical, yet often overlooked, elements in their overall fitness. When T cells are tapped out by intense energy demands or exposed to a hostile tumor microenvironment, their performance can be dampened by:
- Insufficient energy production
- Metabolic imbalances between glycolysis and oxidative phosphorylation
- Inadequate nutrient availability in the tumor vicinity
This scenario leads to a state where even a high number of CAR-T cells might not translate into effective anti-tumor action. The challenge is real: finding ways to modulate these metabolic pathways to keep the T cells energetic and fighting fit.
Enhancing Energy Production in CAR-T Cells
Experts suggest several methods of steering through these metabolic twists and turns:
- Modulation of Energy Pathways: Adjusting the balance between oxidative phosphorylation and glycolysis to ensure that T cells have a reliable energy supply during their intense battle against cancer.
- Nutrient Supplementation: Providing necessary nutrients during therapy could help maintain an adequate energy level within the cells.
- Genetic Modifications: Engineering T cells to better handle metabolic stress can be a transformative approach, potentially enhancing their resilience and longevity once reintroduced into the patient’s body.
In many respects, addressing these metabolic issues could be as essential as fine-tuning the genetic and functional properties of the T cells themselves. With improved metabolic fitness, CAR-T cells can achieve greater expansion, persistent responses, and ultimately better tumor control.
Diversity in T Cell Receptors: A Crucial Biomarker
T-cell receptor (TCR) diversity represents one of the less obvious, but no less important, factors influencing CAR-T cell therapy. The wide variability within the TCR repertoire not only affects the way T cells recognize and interact with tumor antigens, but it might also serve as a potential biomarker for predicting patient responses. In essence, having a diverse TCR pool is akin to having a well-stocked toolbox, ready with multiple options to tackle various cancer cells.
Why TCR Diversity Matters
A diverse TCR repertoire means that the immune system has multiple ways to recognize and attack cancer cells. This variety can translate into:
- Better Antigen Recognition: A wider range of TCR structures allows the immune system to spot even subtle differences among tumor antigens.
- Enhanced Adaptability: When confronted with antigen variations due to tumor mutation, a rich TCR diversity equips the immune response with necessary adaptability.
- Indicator of Immune Robustness: A high level of TCR diversity may correlate with a more robust and resilient immune system, potentially leading to better outcomes for patients receiving CAR-T therapies.
This small distinction in TCR variability could be the key indicator for clinicians looking to predict which patients are most likely to benefit from a given CAR-T cell product. In this light, integrating multi-omics profiling techniques can serve as an essential tool to assess and refine patient-specific therapies.
Integrative Approaches for CAR-T Therapy Enhancement
Researchers are beginning to combine TCR diversity analyses with metabolic and genetic profiling to design more robust CAR-T cell products. Some of the emerging strategies include:
- Multi-Omics Profiling: A comprehensive approach that includes genomics, transcriptomics, and metabolomics to provide a detailed map of the T cell landscape.
- Tailored Patient Selection: Screening patients based on their TCR diversity and metabolic profiles ensures that the right candidates receive the most suitable version of the therapy.
- Custom-Crafted CAR-T Cells: Personalized engineering of T cells based on a patient’s unique cellular characteristics can lead to improved persistence and overall treatment effectiveness.
Such integrative methods are emerging as must-have elements in the quest to overcome the confusing bits of T cell dysfunction. They represent a promising direction for the future of personalized immunotherapy in hematological cancers.
Combining Therapies for Improved Outcomes
In the evolving landscape of cancer treatment, combining multiple strategies appears to be the way forward for improving CAR-T therapy outcomes. With the success of therapies targeting CD19, researchers and clinicians are now charting new paths to integrate targeted drugs, immunomodulatory agents, and metabolic enhancers alongside CAR-T cells.
The Case for Combination Therapy
Single-agent therapies have their limitations. By blending various treatment modalities, we can address the tangled issues that arise from T cell exhaustion, senescence, and metabolic constraints. Combination therapy offers several synergistic benefits:
- Enhanced T Cell Fitness: Co-administration of checkpoint inhibitors can lighten the load on T cells, reducing exhaustion.
- Improved Control Over TREG Cells: The addition of agents that temporarily inhibit TREG function may allow CAR-T therapies to work more effectively.
- Boosted Metabolic Activity: Metabolic enhancers can secure a steady energy supply for T cells, improving their tumor-killing capacity.
For instance, reducing the ex vivo expansion period while implementing metabolic modulators ensures that T cells maintain their natural vigor and memory characteristics during the manufacturing process. In many clinical settings, these combination approaches have already shown promise in boosting the overall effectiveness of CAR-T therapies.
The Road Ahead: Opportunities and Challenges
Even as improvements in CAR-T cell therapy continue to show promise, the path forward is loaded with challenges. The need to understand and optimize each aspect of T cell function—from exhaustion and memory to metabolism and receptor diversity—poses several nerve-racking hurdles. Yet, with each challenge comes an opportunity to innovate and refine these therapies for better patient care.
Key Opportunities for Future Research
The emerging landscape is ripe with potential breakthroughs. Some of these include:
- Advanced Genetic Engineering: Leveraging CRISPR and other gene-editing tools to create more resilient CAR-T cells.
- Precision Metabolic Control: Developing new agents that optimize T cell energy production without triggering adverse effects.
- Multi-Omics Integration: Employing holistic profiling approaches to tailor CAR-T production to individual patient needs.
- Biomarker Discovery: Exploring TCR diversity and other subtle indicators as predictors of therapeutic success.
By embracing these lines of inquiry, the medical community is taking a closer look at some of the tricky parts of immune modification. Each advancement is a step toward ensuring that patients have access to therapies that are not only effective but also customized to overcome the fine points of their individual disease characteristics.
Challenges in Implementation
Despite the many opportunities, significant barriers remain. Some of the key challenges include:
- Standardizing Manufacturing Processes: The production of CAR-T cells is a meticulous process, and even minor deviations can impact cell functionality.
- Managing Side Effects: The interplay between cellular metabolism, TREG cells, and the immune system can sometimes lead to unintended off-target effects.
- High costs: The financial burden associated with tailored therapies remains a major obstacle for widespread clinical application.
- Regulatory Hurdles: Ensuring that new approaches meet strict regulatory standards while still offering innovative solutions is a delicate balancing act.
These challenges call for continued collaboration between clinicians, researchers, and policymakers. With open dialogue and cooperative research, the complex issues associated with CAR-T therapy can be managed more effectively, paving the way for greater accessibility and improved patient outcomes.
Table: Key T Cell Characteristics and Their Impact on CAR-T Therapy
The table below summarizes the critical T cell characteristics and outlines their respective influences on the success or limitations of CAR-T cell therapy:
| T Cell Characteristic | Impact on CAR-T Therapy | Potential Intervention |
|---|---|---|
| T Cell Exhaustion | Reduces proliferation and tumor-killing ability | PD-1 blockade, CRISPR gene editing, optimized expansion techniques |
| Memory Differentiation | Determines persistence and durability of response | Select early memory T cell subsets (TSCM, TCM) |
| Senescence | Limits cell proliferation and activity | Telomerase activation, senolytic agents, pre-treatment optimization |
| TREG Cells | May suppress the overall anti-tumor response | Selective inhibition, targeted depletion, engineered resistance |
| Metabolic Function | Essential for energy supply and proliferation | Metabolic modulators, nutrient supplementation, tailored genetic modifications |
| TCR Diversity | Improves antigen recognition and immune adaptation | Multi-omics profiling, biomarker-based patient selection |
This organized summary helps to figure a path through the various factors that need to be considered when optimizing CAR-T therapies for hematological malignancies. Each intervention not only addresses one of the many small distinctions of T cell behavior but also contributes collectively to improved outcomes.
Expert Opinions and the Future of CAR-T Therapy
Across the globe, experts in immunology and oncology are united in their view that the future of CAR-T cell therapy lies in addressing these individual yet intertwined factors. The small distinctions in T cell metabolism, the fine points of memory cell dynamics, and the tangled issues associated with T cell exhaustion all contribute to a larger narrative that is gradually being reshaped by scientific advances.
What Oncology Specialists Are Saying
Renowned clinical researchers emphasize that the success of CAR-T therapy will depend on a comprehensive approach that includes:
- Re-evaluating patient selection criteria based on immune profiling
- Integrating metabolic and genetic modification strategies into the manufacturing process
- Implementing combination therapy regimens that mitigate immune suppression by TREG cells
These insights are backed by multi-center studies that have shown improved outcomes when these various elements are fine-tuned in unison. The work being done is not just about finding a cure—it’s about making a practical, patient-oriented solution that considers the full spectrum of challenges inherent to immunotherapy in hematological malignancies.
Looking Ahead: Innovation Through Collaboration
In the near future, the collaboration between biotechnologists, genetic engineers, and clinical oncologists is expected to foster an environment where even the most nerve-racking challenges can be overcome. As more data emerges from ongoing clinical trials and real-world applications, the lessons learned will help steer through the intricate landscape of CAR-T therapy with improved precision and efficiency.
Experts remain cautiously optimistic. They stress that while the journey is full of twists and turns, every incremental improvement in understanding and technology brings us one step closer to making CAR-T therapy a mainstay in the treatment of hematological cancers. This optimism is rooted in the ever-evolving field of gene editing, immunotherapy, and multi-omics profiling—each contributing to a future where personalized medicine becomes not just a goal, but a reality.
Conclusion: Towards a Refined Future of CAR-T Cell Therapy
The ongoing research into the intrinsic properties of T cells offers a beacon of hope for enhancing the efficacy of CAR-T therapies. By assessing T cell exhaustion, harnessing the power of early memory cells, mitigating senescence, and managing the dual role of TREG cells, we are increasingly able to tackle the confusing bits that hinder therapeutic success.
Combined with efforts to boost metabolic processes and preserve TCR diversity, these multi-pronged strategies create a comprehensive roadmap for future innovations in immunotherapy. While challenges remain—ranging from manufacturing standardization to high treatment costs—the path forward is illuminated by advances in genetic editing, metabolic engineering, and personalized treatment regimens.
In summary, the key takeaway is that understanding and manipulating the little details of T cell biology is not simply a matter of academic interest, but a practical necessity to ensure that CAR-T cell therapy becomes more durable, effective, and widely accessible for patients battling hematological malignancies. As healthcare practitioners and researchers continue working through these tangled issues, the evolution of CAR-T therapy will undoubtedly lead to breakthrough treatments that balance immediate tumor eradication with long-term patient remission.
In this rapidly evolving therapeutic arena, the collective insights from multi‑omics profiling and targeted genetic modifications yield a road map that is both innovative and pragmatic. By merging tailored approaches with combination therapies, the medical community is making great strides towards overcoming the nerve-racking challenges that have, until now, impeded the full potential of CAR-T cell therapy.
The future of cancer treatment is promising, and with every new study and clinical trial, we get a little closer to turning these early breakthroughs into long-term cures. Continued investment in research and collaboration across specialties will be critical as we strive to provide not only hope but also effective, sustainable treatments for those in need.
Originally Post From https://www.cancernetwork.com/view/impact-of-t-cell-characteristics-on-car-t-cell-therapy-in-hematological-malignancies
Read more about this topic at
Optimizing CAR-T cell therapy for solid tumors
Optimizing the manufacturing and antitumour response of …