Reevaluating Chemotherapy Options in Advanced Gastric Cancer: A Closer Look at TFOX versus FOLFOX
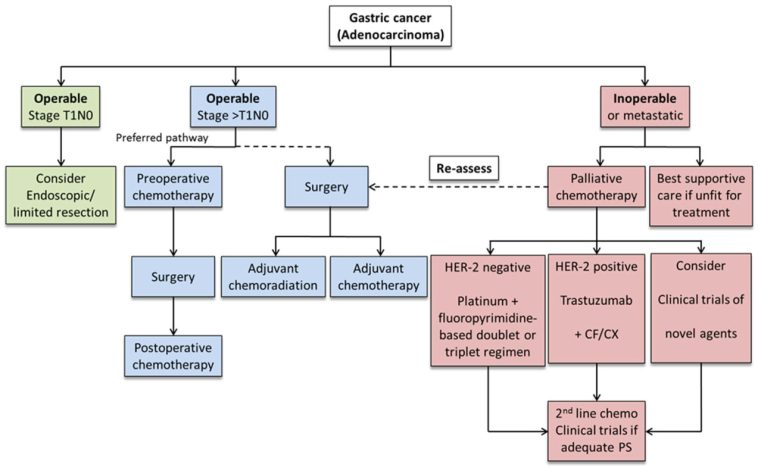
The landscape of advanced gastric cancer treatment is ever-changing as researchers and clinicians work to find the best ways to help patients face this challenging disease. Recent findings from the phase 3 PRODIGE 51-FFCD-GASTFOX trial have prompted many experts to reexamine the therapeutic options available for HER2-negative advanced gastric and gastro-esophageal junction adenocarcinoma. In this opinion editorial, we take a closer look at the modified TFOX chemotherapy regimen and compare it with the established FOLFOX regimen, discussing aspects like progression-free survival, overall survival, objective response rates, and the safety profile using common language to explore each twist and turn in the data.
Advanced gastric cancer remains a significant health issue worldwide. Despite advances in targeted therapies and immunotherapy, chemotherapy continues to be a cornerstone of therapy for many patients. Thus, refining and optimizing chemotherapy regimens is not just a scientific challenge but a key element in improving patient outcomes. With a median follow-up exceeding 42 months in the trial, the data have provided fresh insights into the potential of the TFOX regimen—a combination of docetaxel, folinic acid (leucovorin), oxaliplatin, and 5-fluorouracil—to not only extend survival but also deliver more favorable response rates.
Advanced Gastric Cancer Treatment Landscape
Gastric cancer, along with gastro-esophageal junction tumors, poses many tricky parts and tangled issues that require a multifaceted approach. Traditional chemotherapy regimens, such as FOLFOX, have served as a reliable backbone of treatment for years. However, the need to address some of the confusing bits surrounding survival outcomes and tumor responses has led clinicians to consider more innovative approaches.
The PRODIGE 51-FFCD-GASTFOX trial was designed to address these complex pieces by comparing the well-known FOLFOX regimen with the modified TFOX regimen. With this trial, researchers aimed to figure a path through the little twists and subtle parts of chemotherapy by investigating whether adding docetaxel to the mix could improve the outcomes for patients. For clinicians and patients alike, the possibility of a new therapeutic option that is both effective and manageable in terms of side effects represents a promising step forward.
Exploring the TFOX Chemotherapy Regimen
How TFOX Works and the Potential Benefits
The TFOX regimen is an adaptation of chemotherapy protocols that have been used for several years, updated to include docetaxel. Docetaxel is known to work by interfering with cellular division, thus helping to slow down or stop the growth of cancer cells. In the context of the trial, TFOX was administered every two weeks with a dosage schedule designed to maximize efficacy while keeping side effects under control.
The idea behind integrating docetaxel into the regimen is based on the belief that it might reinforce the anti-tumor activity of the combination. It also challenges the conventional FOLFOX approach, where docetaxel is omitted. By taking a closer look at the fine points of chemotherapy, researchers posited that TFOX could improve progression-free survival and overall survival outcomes, which are super important markers when assessing the success of cancer treatment.
Administration Details and Dosage Comparisons
The treatment protocols for TFOX and FOLFOX, while similar in many ways, have some critical differences that influence how patients experience their treatment. Both regimens are administered intravenously and are continued until there is evidence of disease progression, unacceptable toxicity, or a decision is made by either the investigator or patient to discontinue therapy.
| Parameter | TFOX Regimen | FOLFOX Regimen |
|---|---|---|
| Docetaxel | 50 mg/m2 administration adds an extra dimension to treatment | Not included |
| Folinic Acid (Leucovorin) | 400 mg/m2 | 400 mg/m2 |
| Oxaliplatin | 85 mg/m2 | 85 mg/m2 |
| 5-Fluorouracil (5-FU) Bolus | Not separately noted | 400 mg/m2 |
| 5-Fluorouracil Continuous Infusion | 2400 mg/m2 over 46 hours | 2400 mg/m2 over 46 hours |
As seen in the table, the primary twist here is the addition of docetaxel. Clinicians witnessed that this extra element might be the deciding factor in boosting both the objective response rates (ORR) and disease control rate (DCR) in patients with at least one measurable lesion. By integrating docetaxel, researchers were able to get into key survival outcomes that are essential for advanced gastric cancer treatment.
Assessing Efficacy Through Progression-Free and Overall Survival
Digging into Progression-Free Survival Outcomes
One of the primary outcome measures in the trial was progression-free survival (PFS), which refers to the length of time during and after treatment that a patient lives without the cancer worsening. In the trial, patients treated with TFOX experienced a median PFS of approximately 7.59 months compared with 5.98 months for those treated with FOLFOX. This may not seem monumental at first glance, but the difference is statistically significant and speaks to the potential of TFOX to make a measurable impact in the treatment journey of patients.
To put this into perspective, think of the improvement as finding your way through a maze—each additional month without progression is like having a few more clues that help you circumvent some of the confusing bits and nerve-racking challenges of advanced cancer management. While these numbers must be considered alongside other factors, they offer encouragement to both clinicians and patients that improvements in PFS might be achievable with modifications in the treatment plan.
Diving into Overall Survival Benefits
Overall survival (OS) is another critical measure that determines the length of time patients remain alive following their treatment. In the trial, the TFOX group exhibited a median OS of 15.08 months compared to 12.65 months in the FOLFOX group. Moreover, additional survival rate analyses, such as those at 12 and 18 months, further emphasize the potential benefits of TFOX. The 12-month OS rate in the TFOX arm was nearly 60%, significantly higher than the just over 52% reported in the FOLFOX arm, while the 18-month OS rate also favored the TFOX group.
This improvement in OS is not just a statistical artifact. It represents real hope for patients who are grappling with a disease that is loaded with challenges. By extending the overall survival, TFOX may offer patients additional time to seek alternative treatments, spend quality moments with loved ones, and plan for a future that is a bit less overwhelmed by the disease.
Objective Response and Disease Control: A Closer Look
Understanding Objective Response Rates (ORRs)
Objective response rates (ORRs) are an important measure to ascertain how well a tumor is responding to treatment. In the trial, the ORR was observed to be 62.3% in the TFOX group, compared with 53.4% for the FOLFOX group. This means that a higher percentage of patients experienced a measurable reduction in tumor size when treated with the modified TFOX regimen.
When we poke around the data, we notice that this improvement is achieved without unveiling any new or unexpected safety signals. Such an enhancement in response rates presents a valuable objective benefit, especially for patients whose conditions are already entangled with multiple treatment challenges. In practical terms, higher ORRs suggest that the modified regimen may be more effective in taming some of the malignant behaviors of cancer cells.
Evaluating the Disease Control Rate (DCR)
Disease control rate (DCR) is another crucial endpoint that includes the overall proportion of patients who experience either a reduction or stabilization in tumor size. In this trial, the TFOX regimen achieved a DCR of nearly 87%, compared to 78% in the FOLFOX arm. Such a difference can be the deciding factor in clinical decision-making, particularly in cases where the disease might be resistant to other treatment modalities.
For many patients, a higher DCR means that the disease is managed more effectively, allowing clinicians to step in and adjust treatment plans in a way that minimizes the confusing bits and nerve-racking uncertainties associated with advanced gastric cancer. It signifies a greater chance of controlling the aggressive progression of the tumor, thereby adding another feather to the cap of the TFOX regimen.
Key Insights from the PRODIGE 51-FFCD-GASTFOX Trial
Trial Design and Patient Population
The PRODIGE 51-FFCD-GASTFOX trial was a multicentre, randomized phase 3 study designed to compare the effectiveness of the TFOX and FOLFOX regimens in a balanced and systematic manner. Patients were randomly assigned in a 1:1 ratio, creating two well-matched arms for the study. This careful design ensured that the differences in outcomes could be more reliably attributed to the treatment regimen rather than other unpredictable factors.
The trial enrolled a predominantly male population, with most patients having an ECOG performance status of 1. The primary tumor was typically located in the gastro-esophageal junction, and a notable portion of patients did not present with signet ring cell carcinoma. The distribution of the intestinal type of Lauren classification and the presence of liver and lymph node metastases provided a comprehensive backdrop for evaluating the response to treatment. These demographic and clinical characteristics add further nuance to the study’s findings, allowing physicians to figure a path through the various treatment scenarios presented by this patient population.
Subgroup Analyses and Their Implications
One of the intriguing aspects of the trial was its post hoc subgroup analysis. In patients younger than 70 years, those with an ECOG performance status of 0, or those with a diffuse histological subtype, the TFOX regimen showed a clear overall survival advantage. The finer details or small distinctions observed in these subgroup analyses highlight that individual patient factors can significantly influence how effective a therapy is.
These findings are particularly relevant because they suggest that the modified TFOX regimen might be tailored to specific patient populations. For instance, younger patients or those with a better overall health status might derive more benefit from TFOX compared to traditional FOLFOX. It is this sort of subtle part of the data that offers clinicians the opportunity to take a closer look at each patient’s unique case and decide on the most appropriate course of treatment.
Safety and Tolerability: Weighing the Risks and Rewards
Examining Treatment-Emergent Adverse Effects
When introducing a new or modified treatment into clinical practice, safety is as critical as efficacy. In the PRODIGE 51-FFCD-GASTFOX trial, treatment-emergent adverse effects (TEAEs) were reported in nearly all patients. Despite this close-to-universal occurrence, the profile of adverse effects was consistent with what has been documented in previous studies, and no new safety issues emerged despite the addition of docetaxel.
However, there were a few areas that deserve close attention. Among the most frequently reported grade 3 or 4 adverse effects were:
- Peripheral neuropathy – observed in 32% of TFOX-treated patients vs 20% in the FOLFOX group
- Neutropenia – affecting 27% in the TFOX arm compared with 18% in the FOLFOX arm
- Fatigue – seen in 16% versus 8% respectively
- Diarrhea – reported in 15% compared with 7% respectively
These differences underscore the fact that while TFOX may offer enhanced survival outcomes, it also comes with a higher likelihood of certain side effects. In other words, the enhanced aggressiveness of the TFOX regimen means that physicians must be alert and prepared to manage these extra challenges. It’s a bit like stepping into a new territory where the rewards are promising yet require careful handling to avoid pitfalls.
Managing Dose Reductions and Serious AEs
The trial also noted that dose reductions were required in 77% of patients undergoing TFOX compared to 65% in those on FOLFOX. Additionally, serious treatment-related adverse events were more frequent in the TFOX arm (27% vs 13%), primarily due to gastrointestinal issues. These statistics reveal the nerve-racking parts of adapting a more potent chemotherapy regimen. Nonetheless, the overall conclusion was that the safety profile of TFOX remained within an acceptable range, especially when weighed against the potential benefits in terms of PFS and OS.
Clinicians must therefore balance the improved efficacy outcomes with the increased risk of adverse effects. This balancing act is filled with twists and turns. By keeping an eye on the detailed records of side effects and being ready to adjust dosages, healthcare providers can work through these challenges and steer through the complicated pieces that come with an aggressive treatment plan.
Clinical Implications and Future Directions
Integrating TFOX into Standard Care Practices
The emerging evidence suggests that TFOX might represent a new therapeutic option for patients who are not candidates for immune checkpoint inhibitors or targeted agents. Given that not every patient is eligible for these newer forms of therapy, the modified chemotherapy regimen can serve as a critical alternative.
From a clinical standpoint, the key challenge is to find your way through the fine points implicated by the increased objective response rate and extended overall survival. When deciding on a treatment strategy, physicians must consider:
- The patient’s age and overall performance status
- The histological characteristics of the tumor
- The balanced risks related to treatment-emergent adverse effects
By adopting TFOX selectively for patients who are likely to benefit the most, clinicians can maximize therapeutic outcomes while managing the potential side effects. This tailored approach is essential in modern oncology care, where every patient’s case carries its own set of challenges and hidden complexities.
Opportunities for Combining TFOX with Novel Agents
Another exciting avenue to explore is the potential for combining the TFOX regimen with emerging agents such as PD-1 inhibitors or claudin 18.2 inhibitors. Since the trial hints at a more effective chemotherapy backbone using TFOX, future studies could build on this by integrating immunotherapy or targeted treatments with it. This multimodal approach could address some of the subtle details that remain unresolved by conventional treatments alone.
Ongoing and future clinical trials might soon figure a path through these complicated pieces, investigating whether such combinations can further extend survival while keeping adverse events within manageable limits. For patients, this represents a ray of hope amidst a field that remains on edge due to the tense challenges of effectively controlling advanced gastric cancer.
Understanding the Broader Context in Oncological Care
The Real-World Impact on Patient Quality of Life
When discussing any treatment regimen, it’s essential to consider the impact on patients’ quality of life. With TFOX showing an improved median overall survival and progression-free survival, patients potentially gain more time with effective disease management. However, the increased rate of certain adverse effects—such as peripheral neuropathy and fatigue—requires that clinicians take extra care in monitoring and managing these issues.
Quality of life assessments must weigh both the extended life expectancy and the degree to which side effects interfere with daily activities. In scenarios where managing these challenging symptoms becomes a significant part of the treatment, clinicians and patients need to work together to dodge the nerve-racking aspects of chemotherapy while maximizing its benefits.
Practical Considerations for Healthcare Providers
Healthcare providers must figure a path through the practicalities of instituting TFOX as a treatment alternative. This involves:
- Careful patient selection by considering age, performance status, and tumor biology
- Vigilant monitoring of adverse effects, particularly the gastrointestinal and hematologic toxicities
- Adjusting dosages as necessary to strike the right balance between efficacy and tolerability
- Educating patients about both the potential benefits and risks associated with the regimen
These responsibilities underline the importance of an integrated care model where oncologists, nurses, dietitians, and other healthcare professionals work in tandem to support the patient throughout their treatment journey. The collaborative effort required is a reminder that modern oncologic care is more than just a set of isolated treatment protocols—it’s about finding your way together through a series of complex decisions and unexpected challenges.
Patient Perspectives and Decision-Making in Advanced Gastric Cancer
Empowering Patients with Clear Information
In today’s healthcare environment, patient empowerment is key. Patients facing advanced gastric cancer must have access to clear, understandable information about their treatment options. Data showing the improved progression-free and overall survival with the TFOX regimen can help patients and families make informed decisions. By breaking down the data into digestible bits—such as survival statistics and potential side effects—clinicians can alleviate some of the overwhelming and intimidating aspects of treatment planning.
It is essential to present the information neutrally, ensuring that patients understand both the potential benefits and the risks. When patients feel fully informed, they are in a better position to take part in shared decision-making, which in turn leads to higher satisfaction with their care and possibly even improved adherence to the treatment protocol.
Considering the Emotional and Psychological Toll
The journey through any advanced cancer treatment, including those with newer chemotherapy regimens, has many nerve-racking moments. The data from the TFOX trial not only impact clinical outcomes but also have emotional and psychological dimensions. Patients might find comfort in knowing that there are ongoing efforts to improve treatment efficacy, even if it means grappling with extra complications like increased side effects.
Support systems, including counseling and patient support groups, can help individuals manage the twists and turns associated with both the disease and its treatment. A comprehensive care model recognizes that treating cancer involves not just the fine points of medicine but also providing emotional support tailored to each patient’s unique needs.
Looking Ahead: Research, Innovation, and the Future of Chemotherapy
Innovation in Chemotherapy Regimens
On the research front, the encouraging results from the TFOX regimen are a call to action. Future clinical trials could take a closer look at combining TFOX with other promising therapies such as immunotherapy or molecularly targeted drugs. Adding another layer of treatment could potentially address some of the smaller distinctions within patient subgroups and fine-tune the therapy to individual needs.
For example, exploring innovative combinations might help reduce the intimidating side effects or improve the overall response rate even further. Innovation in chemotherapy is about more than merely adding a drug—it’s about understanding the hidden complexities of cancer biology and designing treatment protocols that are as dynamic as the disease they aim to combat.
Collaborative Research and Multidisciplinary Approaches
The evolving treatment options for advanced gastric cancer underscore the importance of a multidisciplinary approach in oncology. Researchers, oncologists, pharmacologists, and patient advocates need to take a closer look at both the efficacy data and the subtle details of patient experience when evaluating new regimens. The insights gathered from trials like PRODIGE 51-FFCD-GASTFOX serve as a springboard for more detailed investigations.
In addition, collaborative initiatives across international research centers can help smooth out the tangled issues of treatment variability. By pooling resources and data, researchers can work through the challenging parts to develop guidelines that are robust and adaptable. Such collaborations are key to addressing the many subtle parts that contribute to the success or failure of treatment regimens in advanced cancer care.
Final Thoughts: Striking the Right Balance in Cancer Care
Balancing Efficacy and Tolerability
The PRODIGE 51-FFCD-GASTFOX trial brings to light several fine shades in the ongoing debate over the optimal frontline chemotherapy regimen for HER2-negative advanced gastric and gastro-esophageal junction adenocarcinoma. On one side, the TFOX regimen offers the promise of improved progression-free and overall survival, along with increased objective response and disease control rates. On the other side, the enhanced potency of the TFOX regimen comes with a higher likelihood of certain severe side effects.
For clinicians, the decision to adopt or recommend TFOX over FOLFOX involves steering through these complicated pieces with care. It means taking a closer look at each patient’s individual profile, understanding the subtle differences in how various subgroups respond to treatment, and ultimately accepting that there is no one-size-fits-all answer. Instead, the optimal strategy lies in a balanced, patient-centered approach that integrates efficacy, safety, and quality of life considerations.
Moving Forward With Cautious Optimism
While it is too early to declare TFOX the new gold standard for advanced gastric cancer treatment, its promising performance in extending both progression-free and overall survival certainly merits further exploration. The trial’s findings encourage oncologists to get into detailed discussions with their patients, weighing the potential benefits against the intimidating risks of higher-grade adverse events.
Ultimately, advancements in chemotherapy regimens like TFOX represent a beacon of hope for many. They remind us that modern medicine is continually evolving, and that by being open to new ideas and new data, we can slowly but surely chip away at the tired, tangled issues that have long plagued cancer treatment. It is this ongoing journey, full of fine details and unexpected challenges, that continues to drive innovation and excellence in healthcare.
Conclusion: The Future of Chemotherapy in Gastric Cancer
The journey of treating advanced gastric cancer is replete with twists and turns. The modified TFOX regimen, with its promising improvements in progression-free survival, overall survival, and response rates, offers a fresh avenue for oncologists to consider. While it is coupled with slightly higher rates of adverse effects, the potential benefits—especially for select patient subgroups—cannot be dismissed.
As we take a closer look at the data and continue to manage our way through the challenges of modern oncology, it becomes clear that no single regimen will serve every patient’s needs. Instead, future progress will likely lie in a personalized approach that considers not only the core effectiveness of a treatment but also its impact on quality of life. Trials like PRODIGE 51-FFCD-GASTFOX provide the much-needed evidence to pave the way for these discussions, allowing patients and clinicians alike to figure out a path that is tailored to the unique hurdles of each cancer journey.
The evolution of chemotherapy is a dynamic story—one that calls for both cautious optimism and relentless pursuit of better outcomes. As researchers continue to explore innovative combinations and as multidisciplinary teams work in tandem to tweak and improve treatment protocols, the ultimate goal remains the same: offering hope, improved survival, and a better quality of life for patients faced with advanced cancer.
In these nerve-racking times, when every month of progression-free survival counts and every improvement in overall survival brings us closer to a future where cancer treatments are as finely tuned as the challenges they aim to overcome, it is heartening to see that progress is not only possible but is actively unfolding. Whether you are a clinician, a patient, or simply someone who believes in the future of healthcare, the ongoing innovations in chemotherapy regimens like TFOX remind us that even in the midst of complicated pieces and overwhelming challenges, hope can be found—and that together, we can figure a path forward into a brighter, healthier future.
Originally Post From https://www.cancernetwork.com/view/tfox-regimen-enhances-efficacy-vs-folfox-in-her2-negative-gastric-cancer
Read more about this topic at
Breakthrough in treatment for stomach and esophageal …
Advances in gastric cancer treatment in 2024